![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


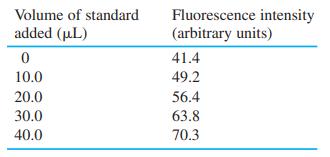
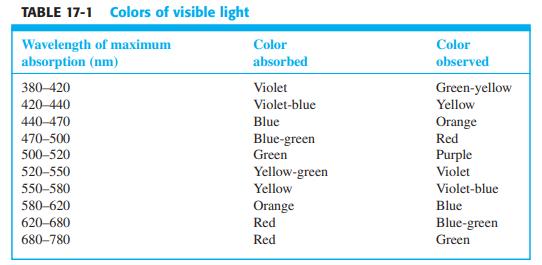
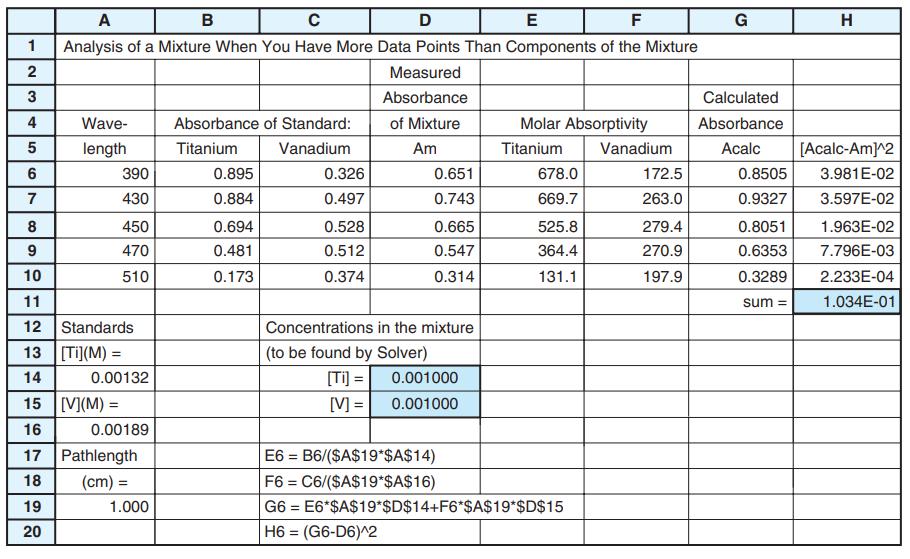
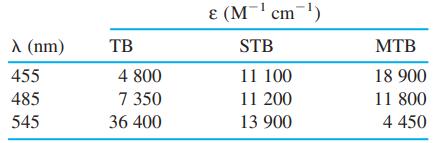
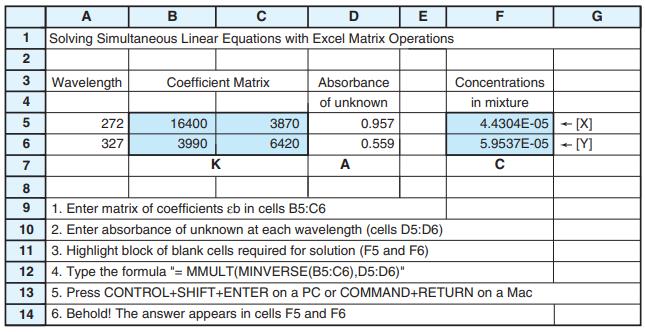
![DE 1 Solving Simultaneous Linear Equations with Excel Matrix Operations A в F G Wavelength Coefficient Matrix Absorbance Concentrations 4. of unknown in mixture 5 16400 4.4304E-05 [X] -[Y] 272 3870 0.957 327 3990 6420 0.559 5.9537E-05 7 K A 9 1. Enter matrix of coefficients eb in cells B5:C6](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/0/1/2685ee0f5b4c96261591801261927.jpg)
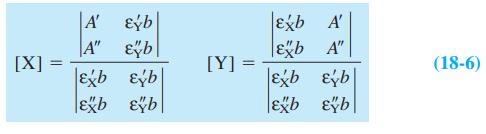
.png)
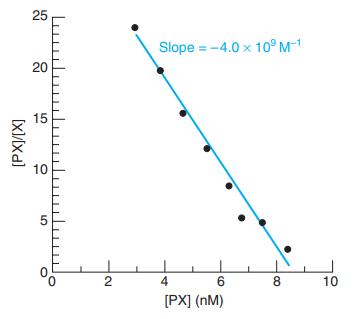
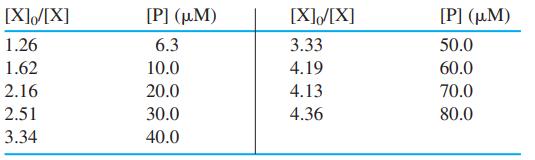
![[PX] [X], - [X] X + P= PX K = %3D [X][P] [X][P]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/1/3/0385ee123ae6ae7f1591813033909.jpg)
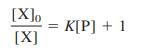
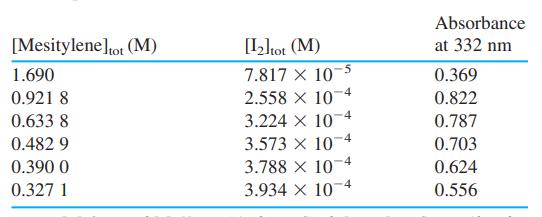
![[complex] I, + K = [I][mesitylene] Iodine Mesitylene Complex Amax = 332 nm E332 E332 0](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/1/3/1625ee1242a369801591813157745.jpg)
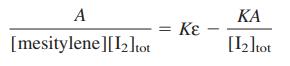
-2.png)
.png)
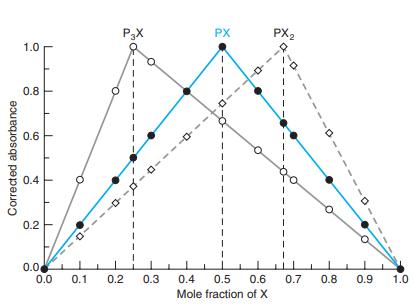
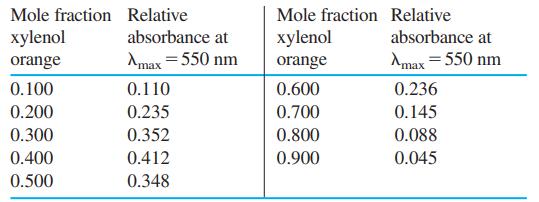
.png)
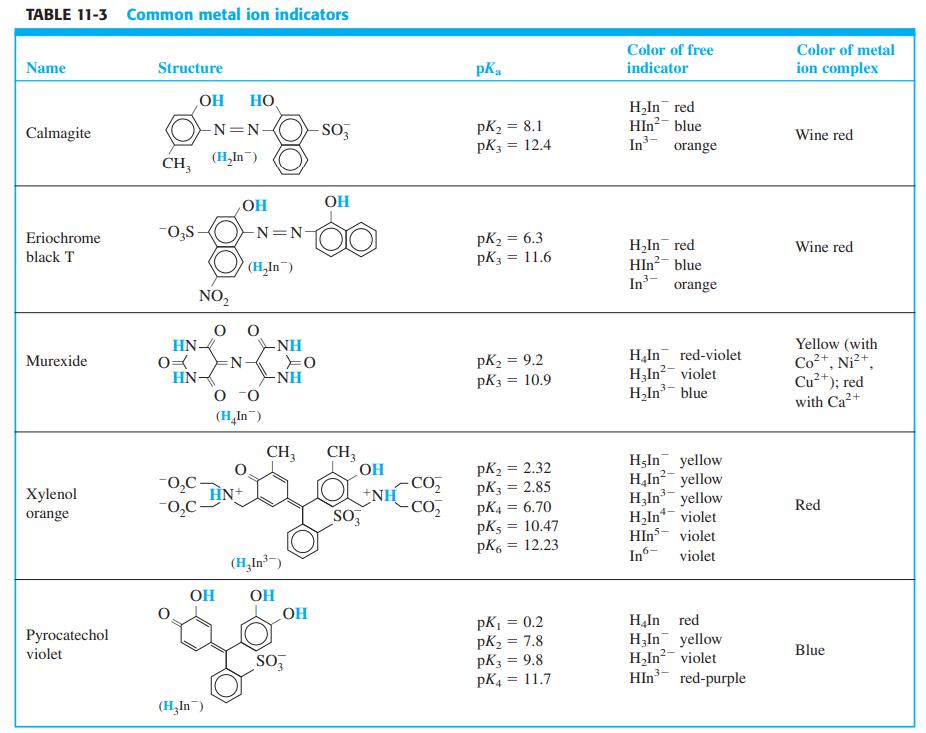
-1.png)
-2.png)
-1.png)
-2.png)
-3.png)
-1.png)
-2.png)
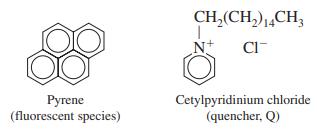
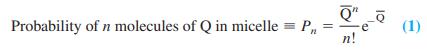
-1.png)
-2.png)
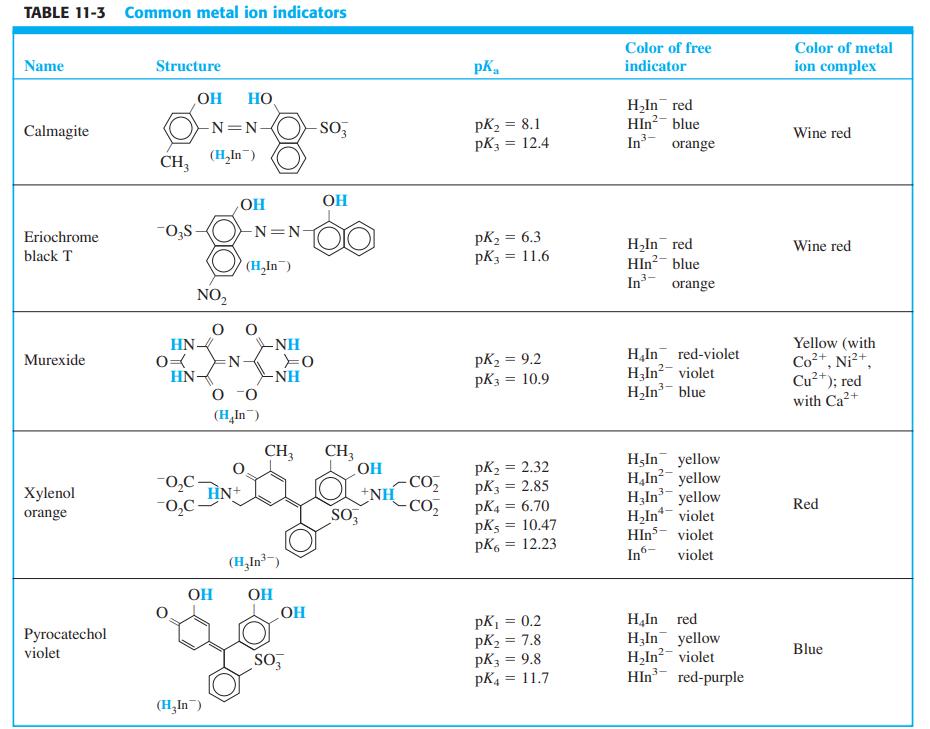
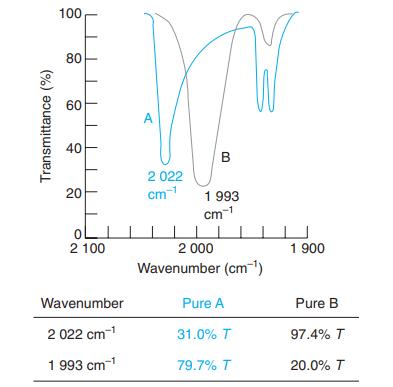
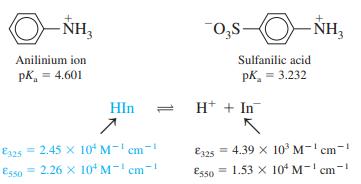
.png)
.png)
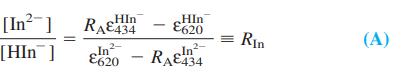
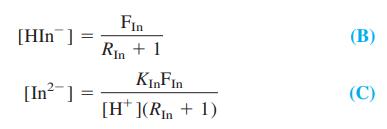
-2.png)
.png)
.png)
.png)
.png)
.png)
.png)
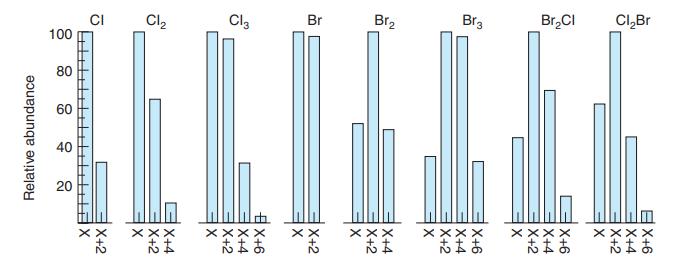
.png)
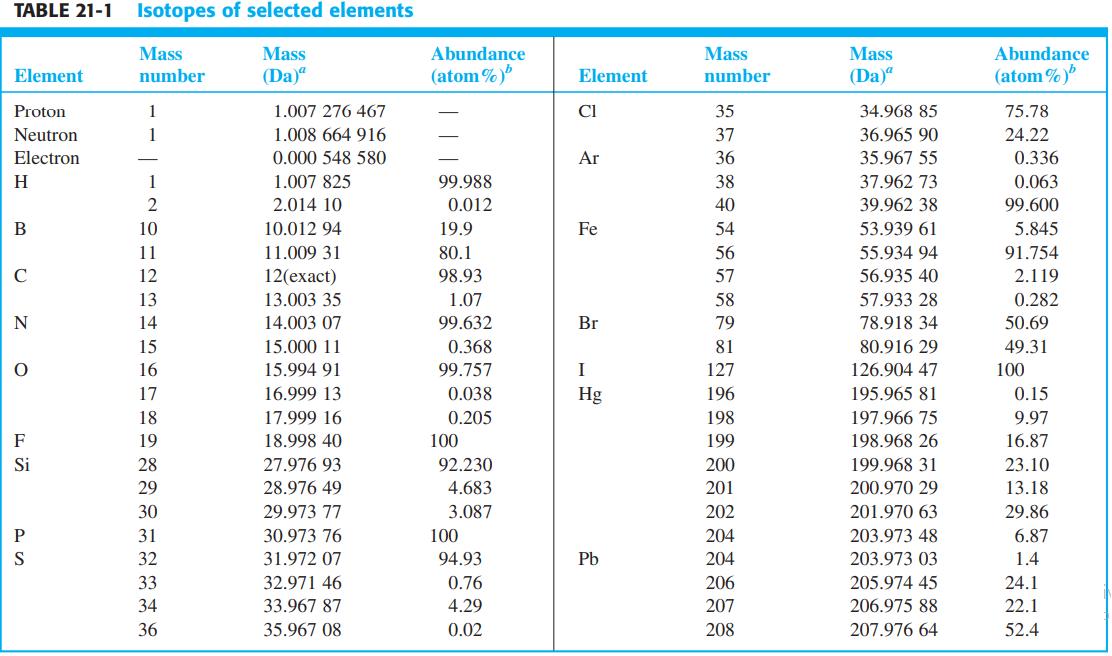
.png)
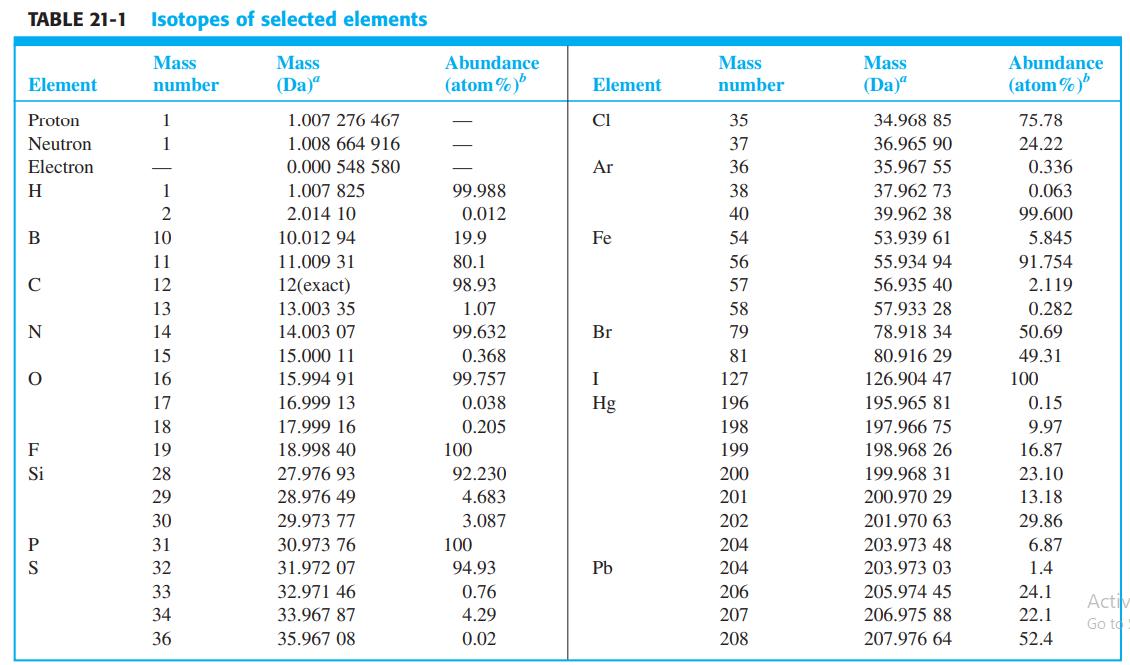
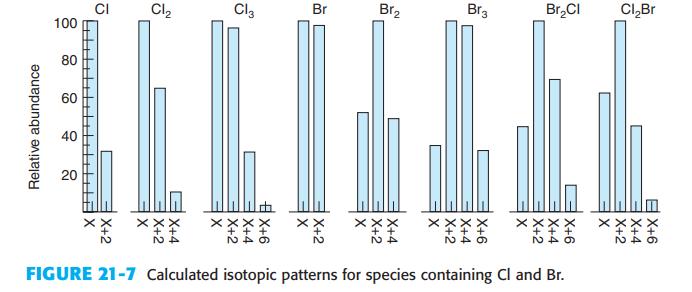
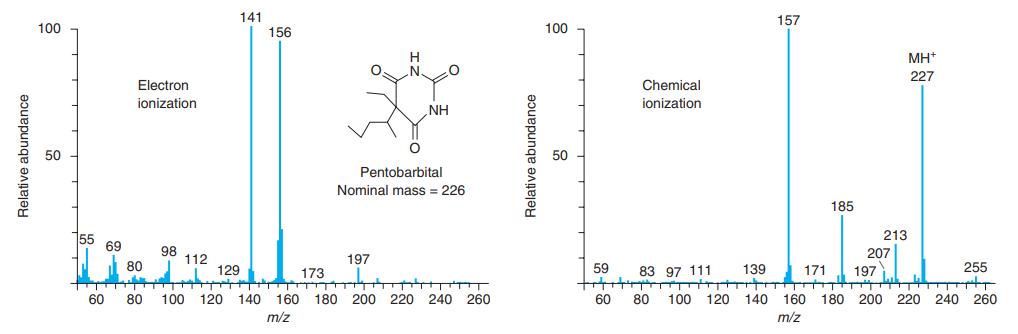
.png)