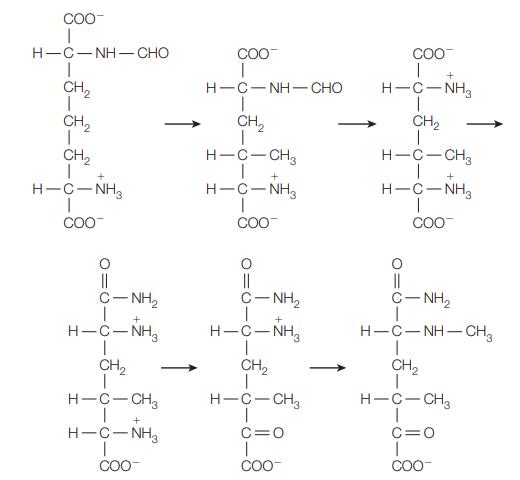
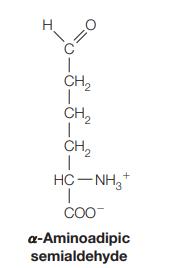
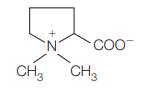
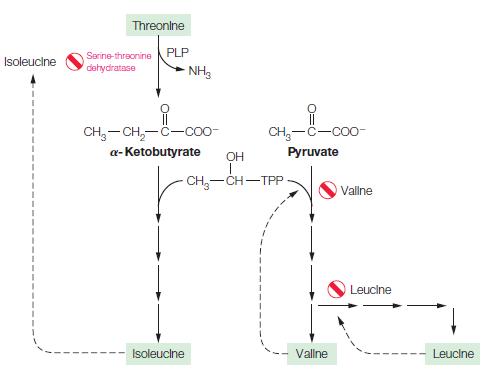
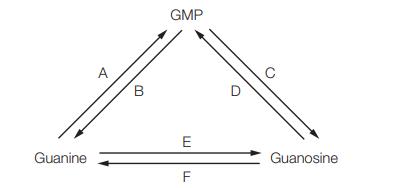
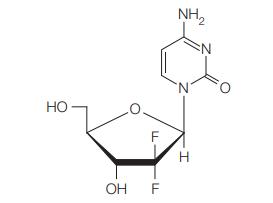
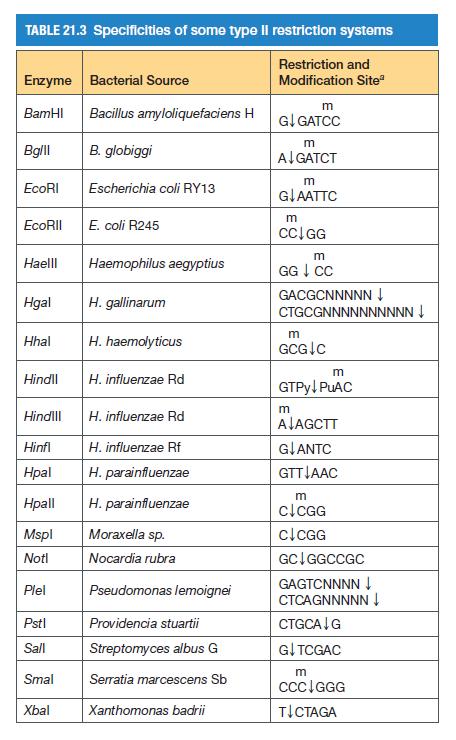
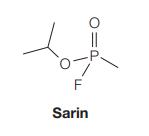
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


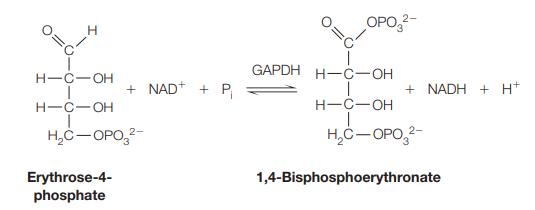
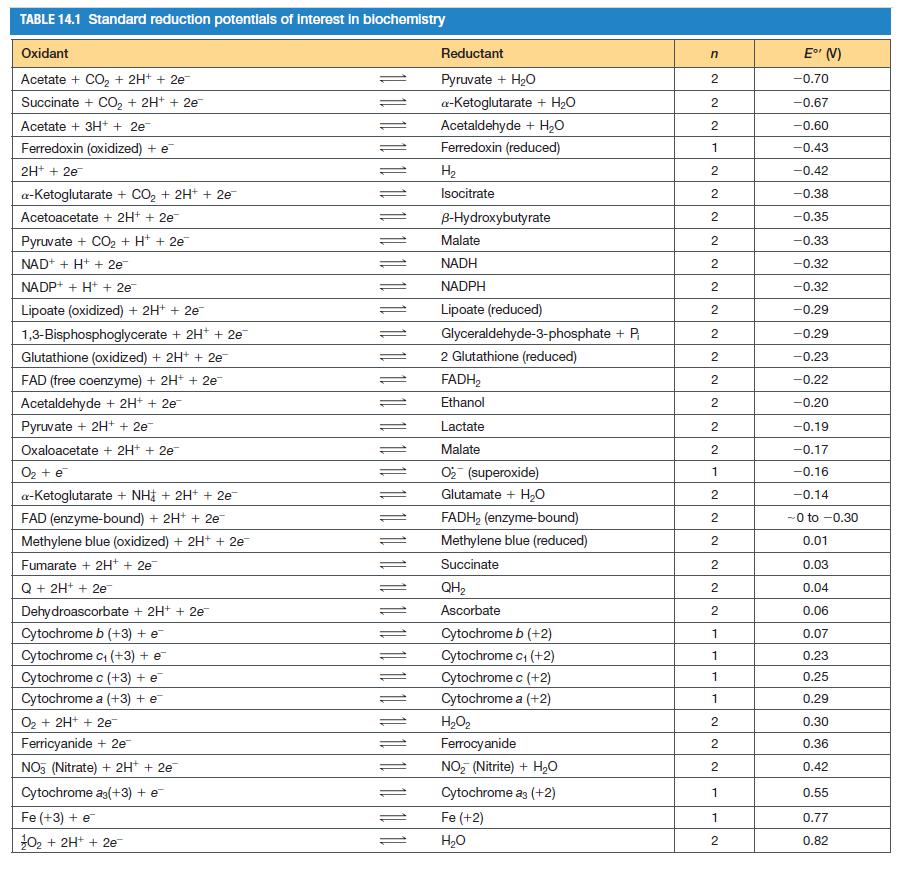
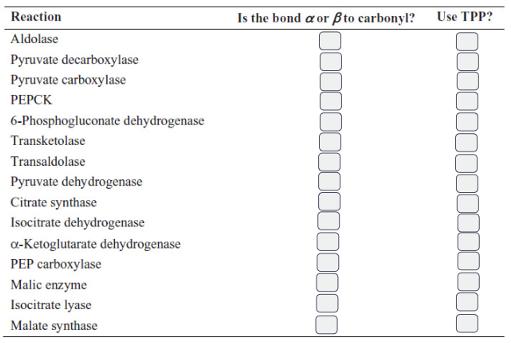
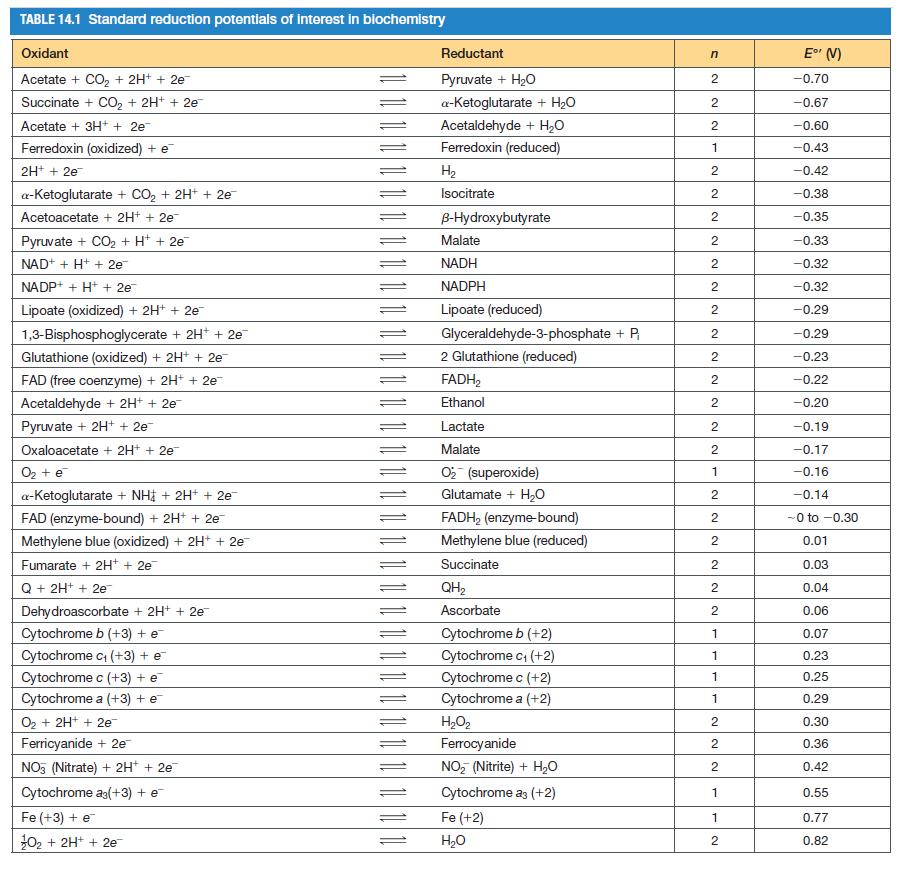
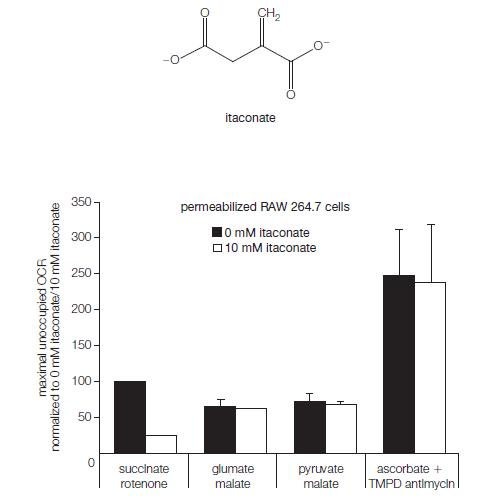
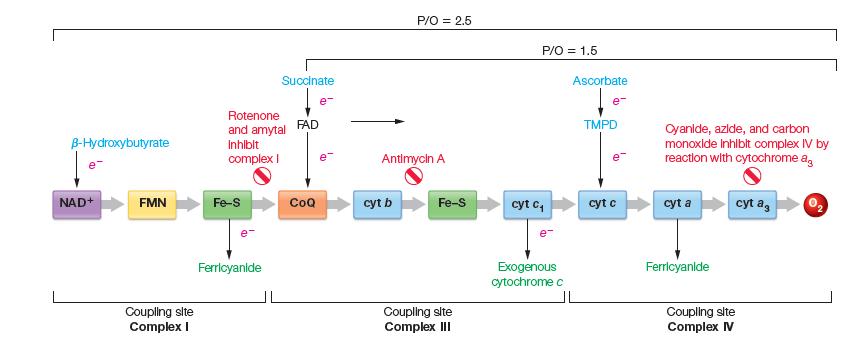
![[CO] (MM) 0.20 0.10 0.067 0.050 Under N 16.7 12.5 8.3 7.1 Under O 10 5.6 4.2 3.2](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/9/9/7/9706544a6d2f405e1698997969265.jpg)