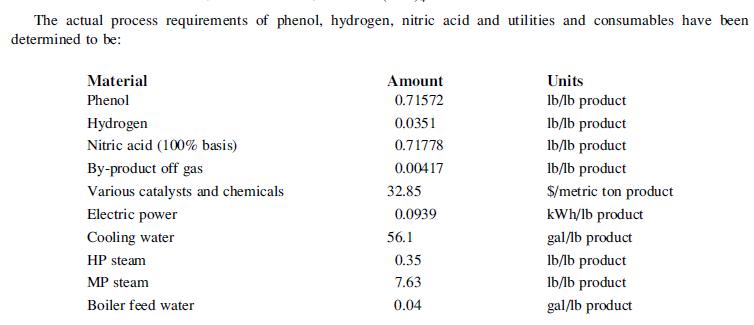
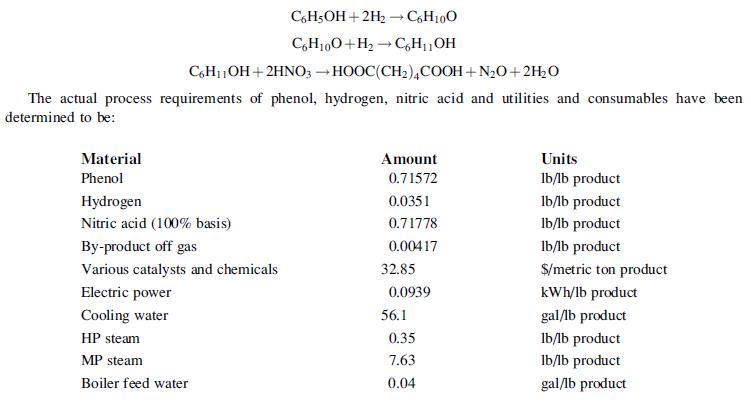
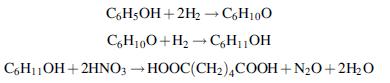![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


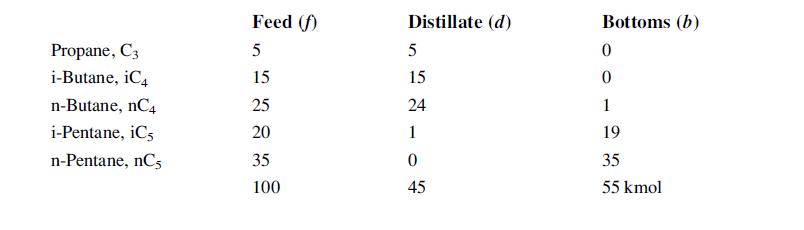
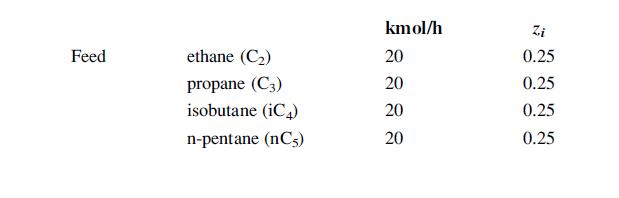
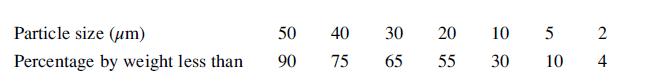
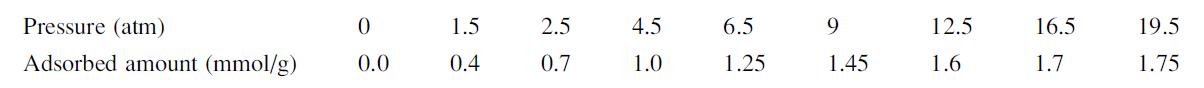
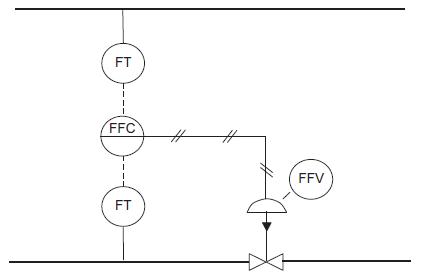
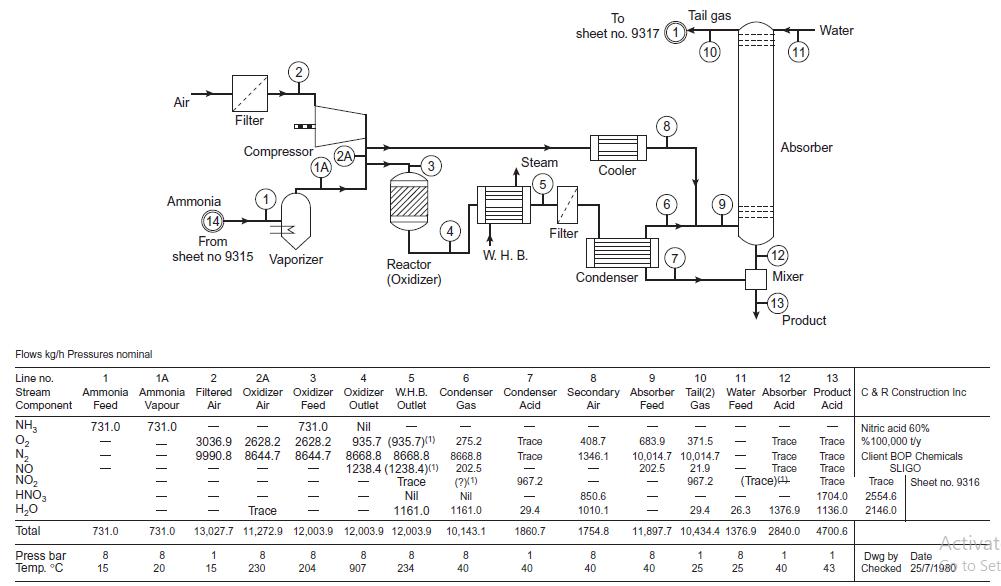
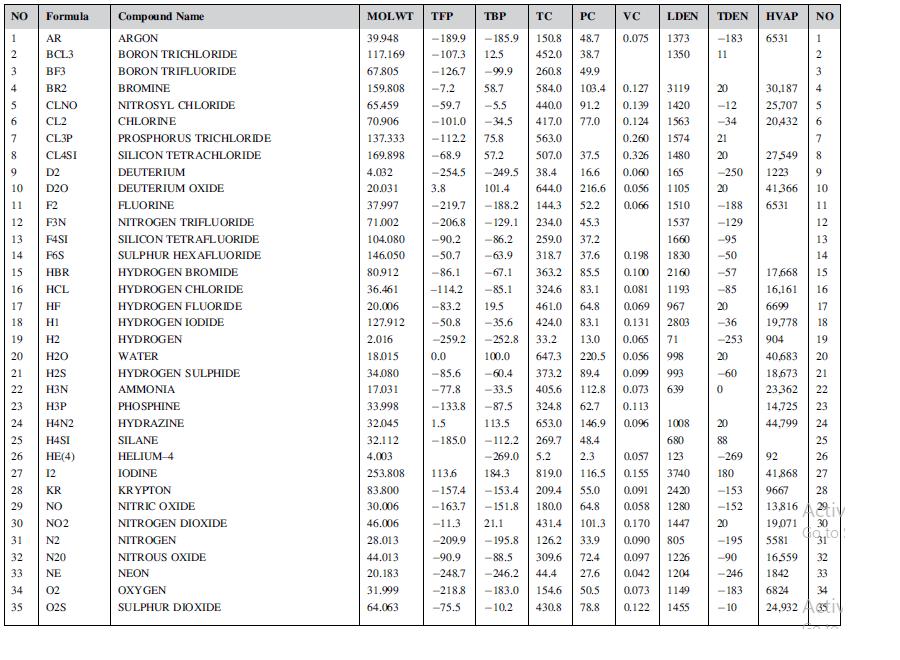
![LOG[viscosity] = [VISA] * [(1/T) - (1/VISB)], viscosity mNs/m, T deg K DELHF = Standard enthalpy of formation](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/0/9/0/03165a19befa8d0d1705090031337.jpg)