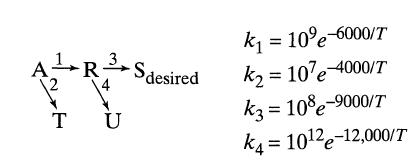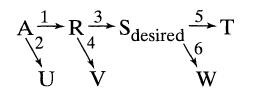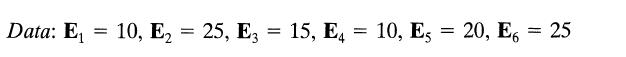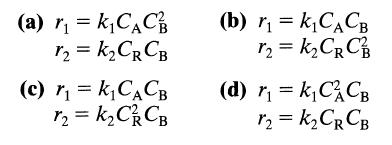![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()



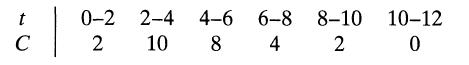
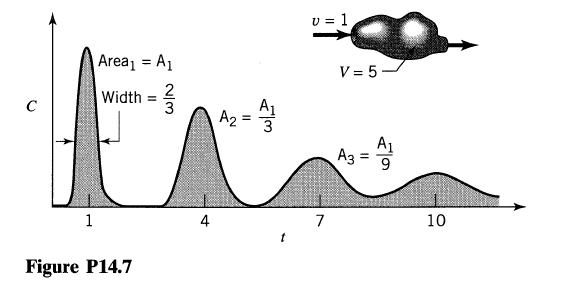
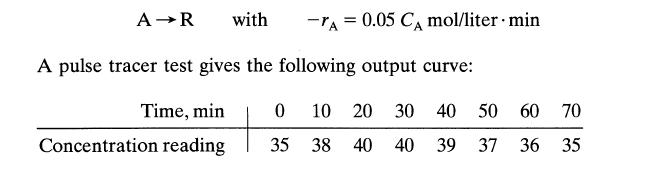
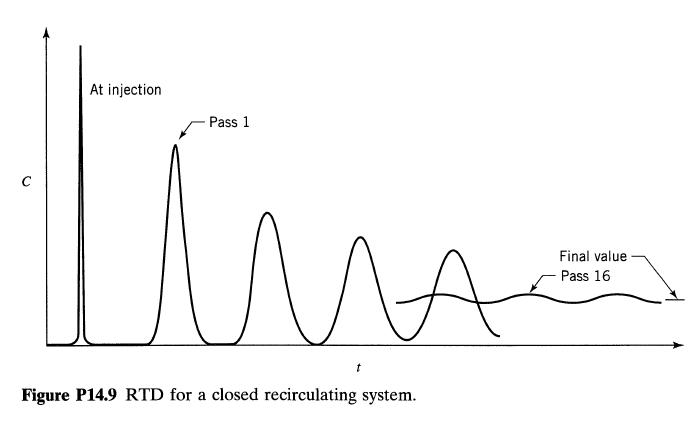

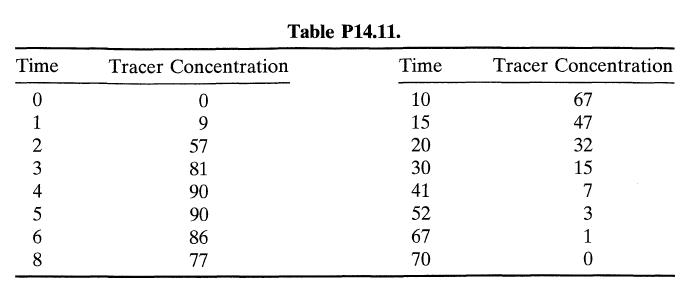
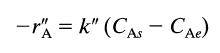
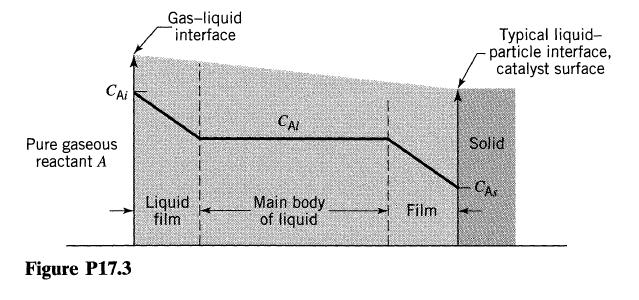
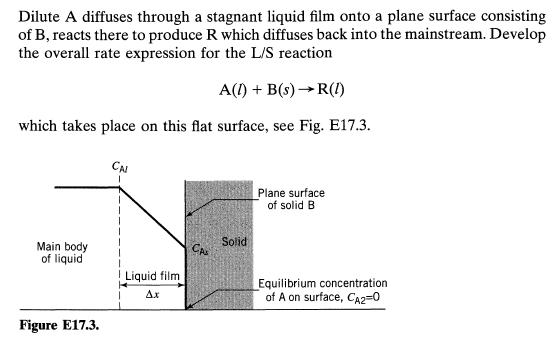
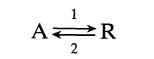
![Data: k = 10 exp [-2416/T] ACp CPR CPA = 0 - AH, = -8000 cal/mol at 300 K K = 10 at 300 K Feed consists of](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/4/0/0/524651d048cabd501696400524239.jpg)