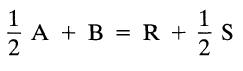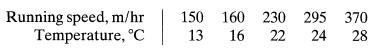![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


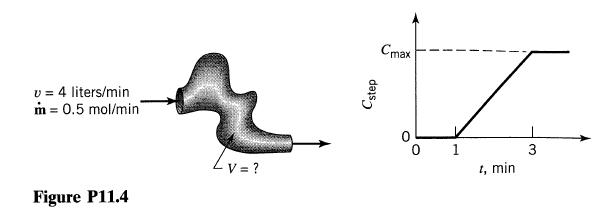

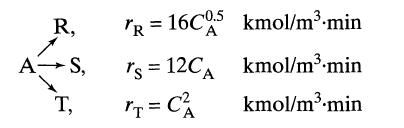
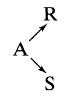

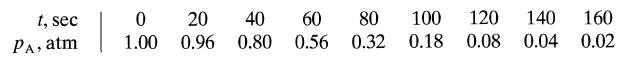
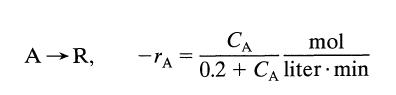
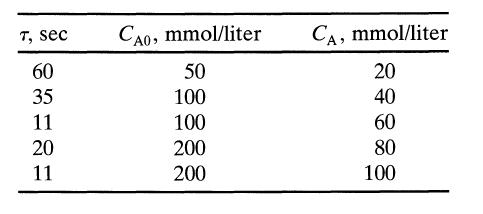
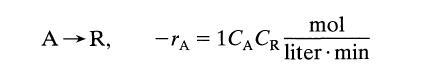
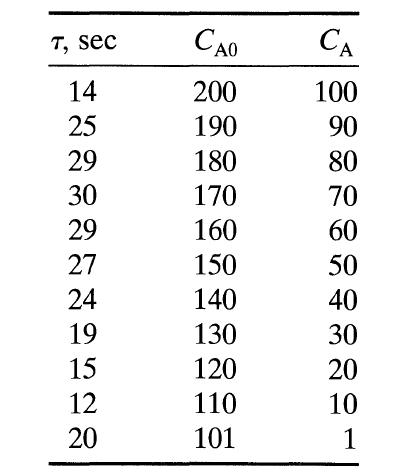
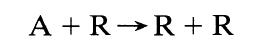
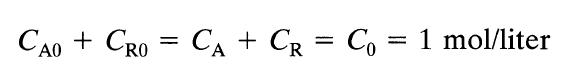
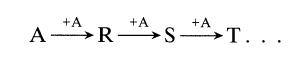
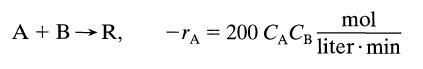
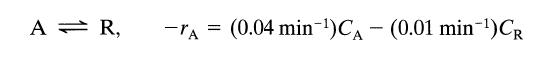
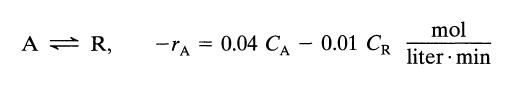
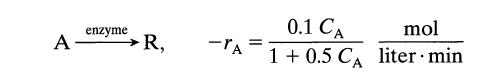
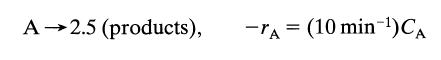
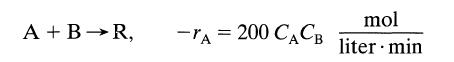
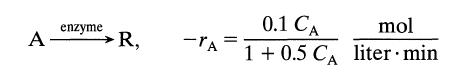

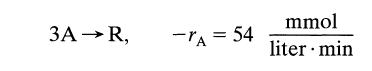
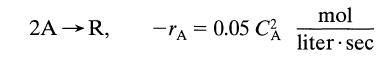
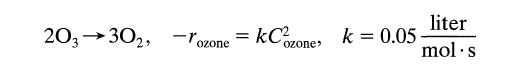

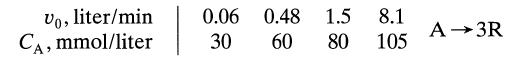
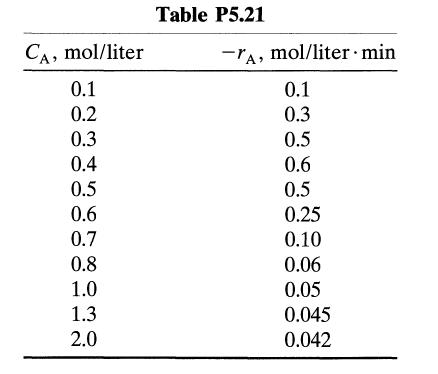
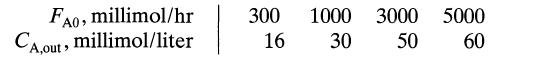
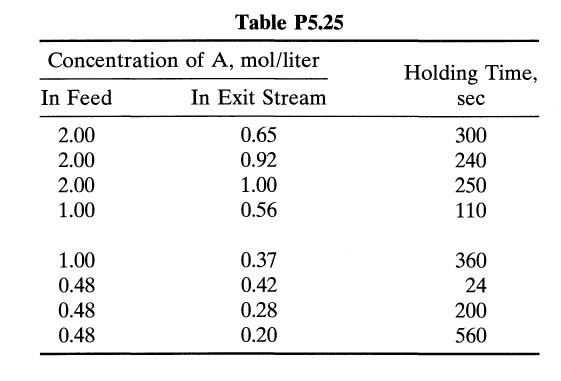
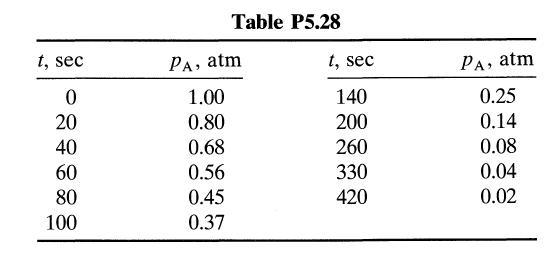
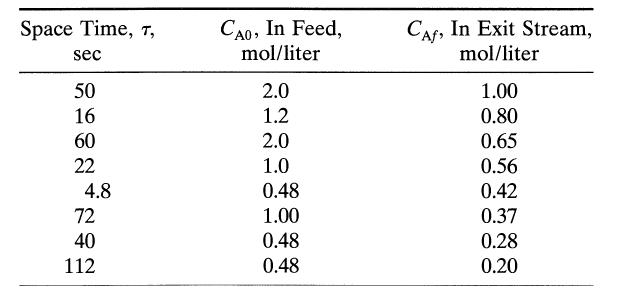
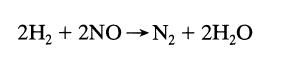

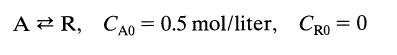
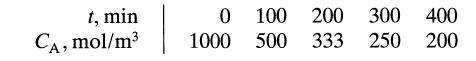
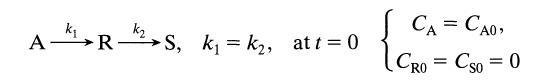
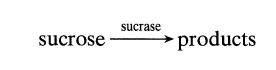

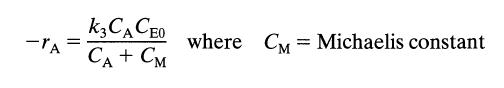
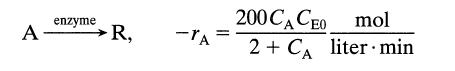
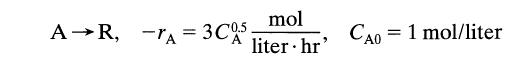
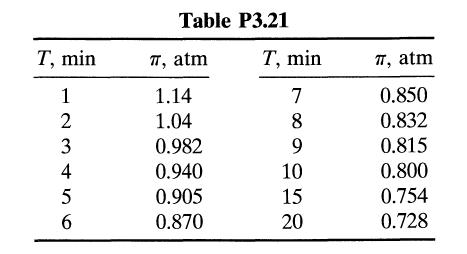

![[A] 1 3 4 2 2 1 Table P3.28 [B] 3 1 4 2 4 2 [C] 0.02 0.02 0.04 0.01 0.03 0.05 TAB 9 and 5 32 6 20 12](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/0/7/0/1336517f9f53f82f1696070129316.jpg)
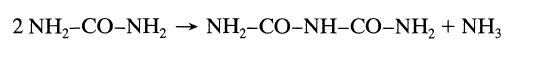
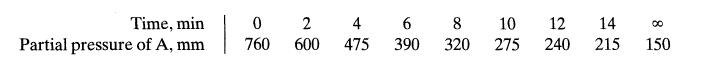

![-TA 1760[A][E] 6+ CA mol/m.s](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1695/9/0/8/86765158403c37911695908865727.jpg)