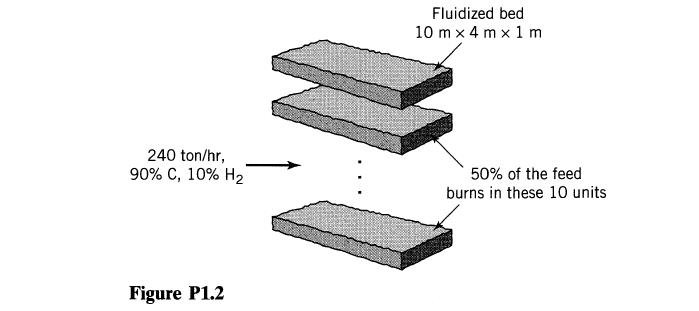
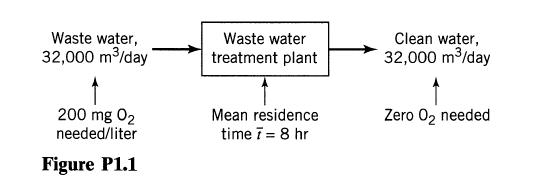
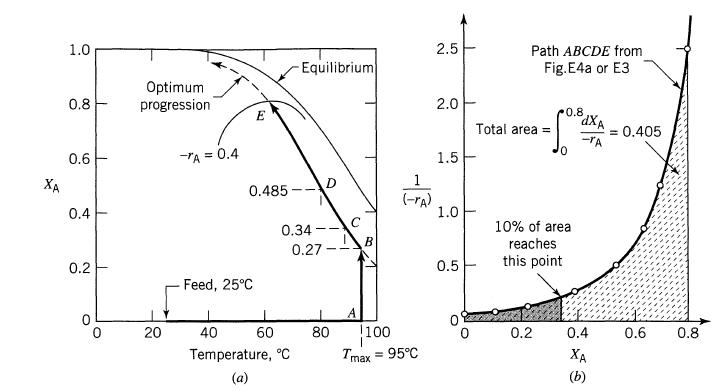
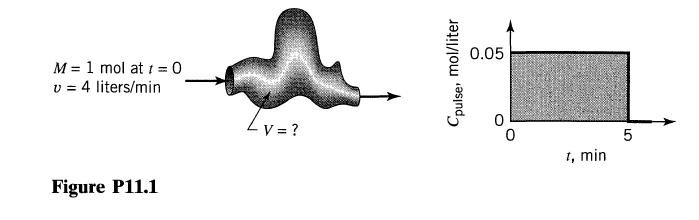
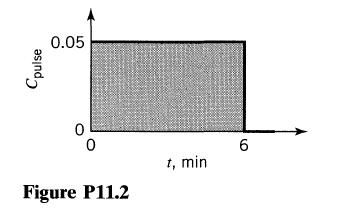
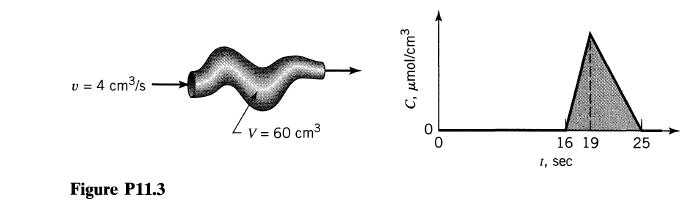
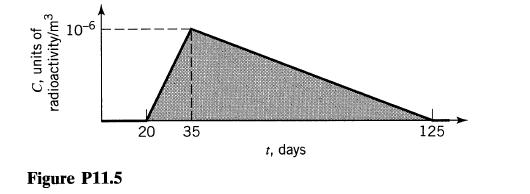
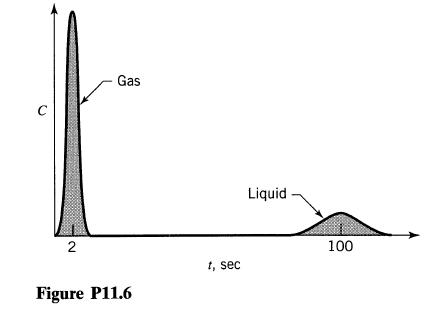
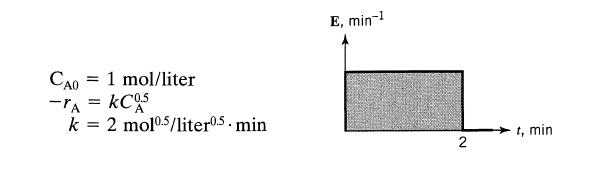
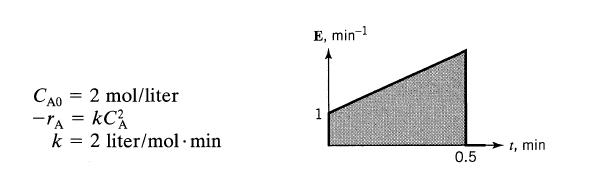
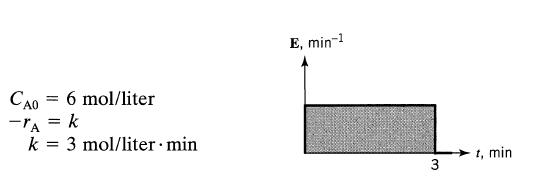
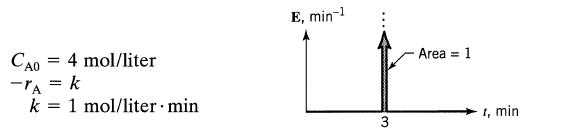
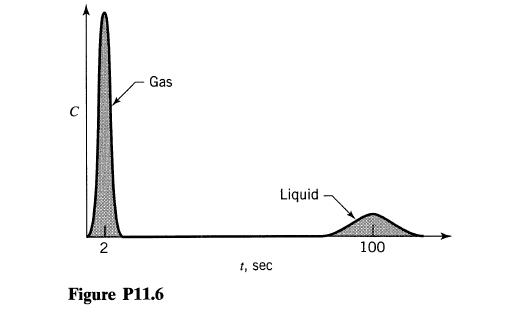
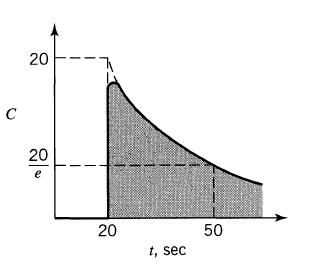
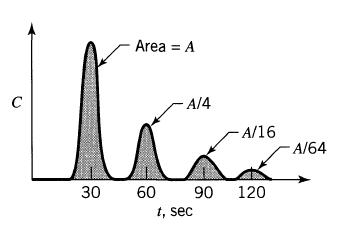
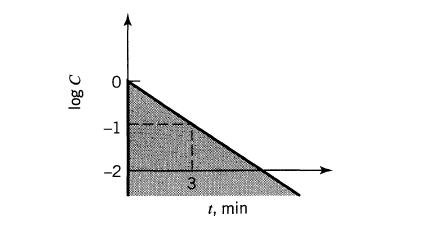
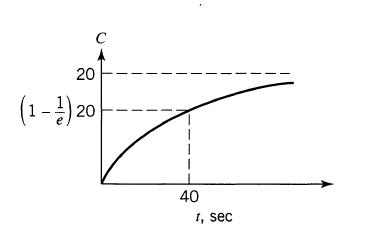
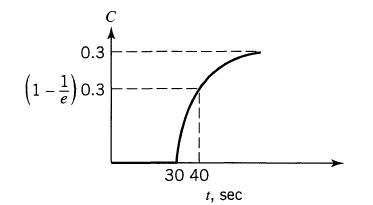
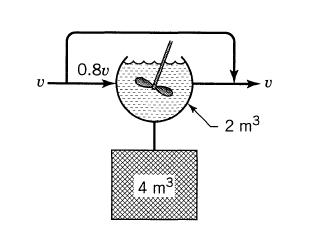
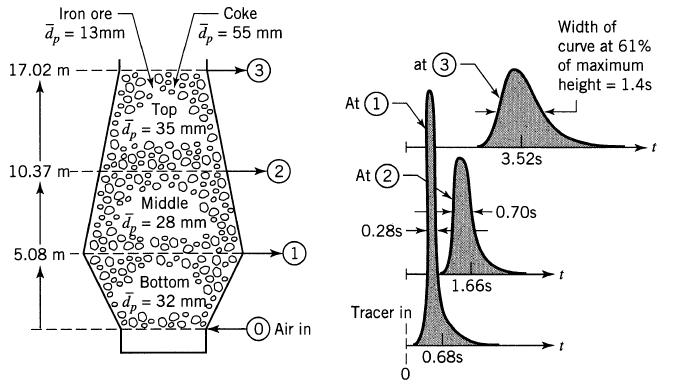
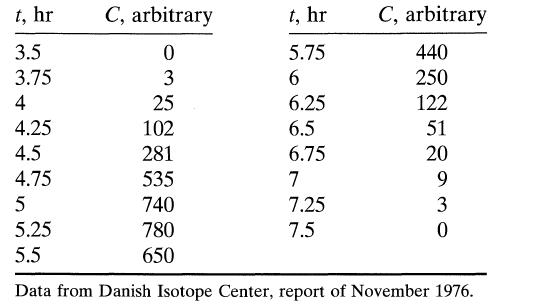
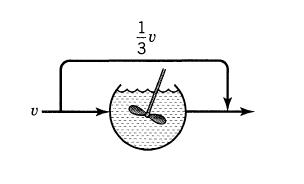
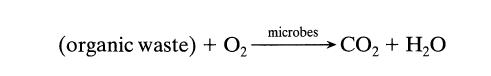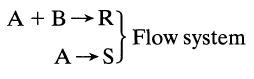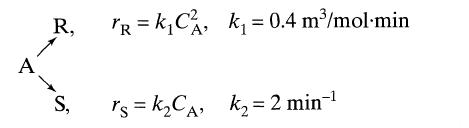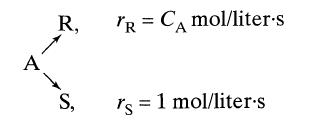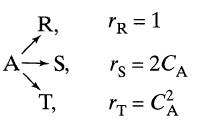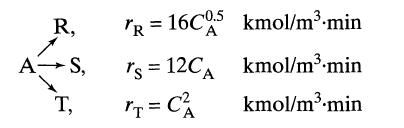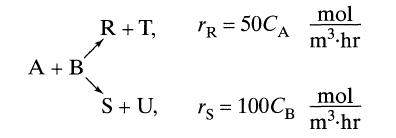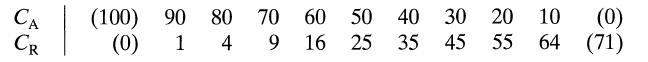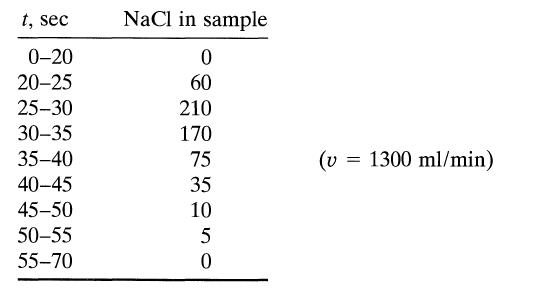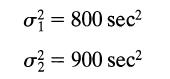![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


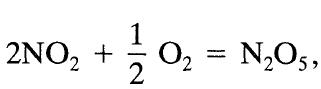
![A+E=X k XR+E with K = [X] [A][E]' and with [E] [E] + [X]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1695/9/1/3/459651595f306d8a1695913456524.jpg)
![k A+EX k k3 X-KR+E with d[X] dt = 0, and [E] [E] + [X] =](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1695/9/1/3/4856515960d79c101695913483256.jpg)
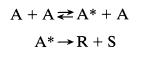
![-ro =k[0] [0]-](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/7/5/5/40165226ec990a421696755401159.jpg)
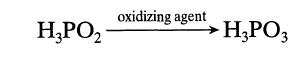
![HPO = k[H+][HPO]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/0/5/7/9856517ca81bf6451696057983265.jpg)
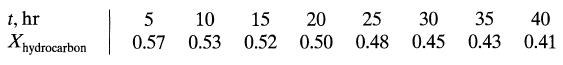

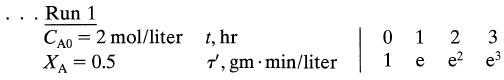

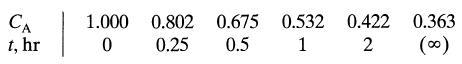
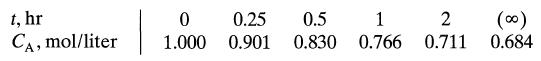
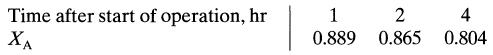
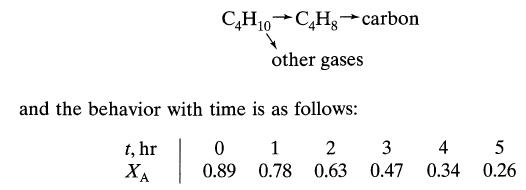
![da dt == Ka(CA+CR) a = 10(CA + CR)a, [day-]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/6/7/4/88565213445684ca1696674880900.jpg)
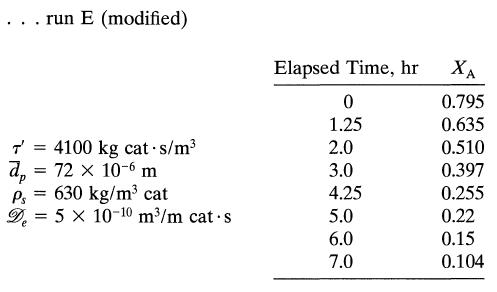
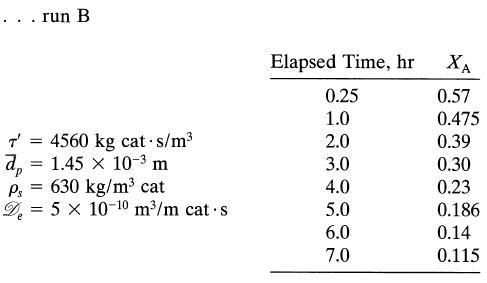
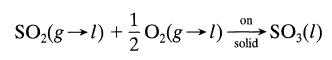
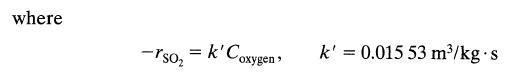
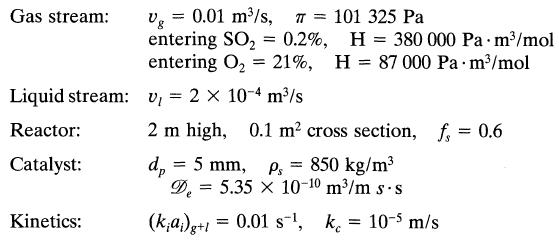
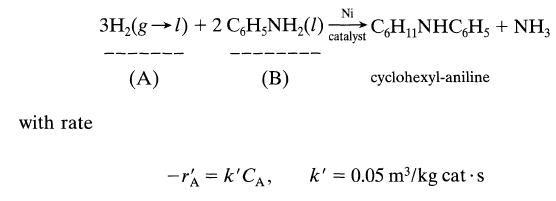
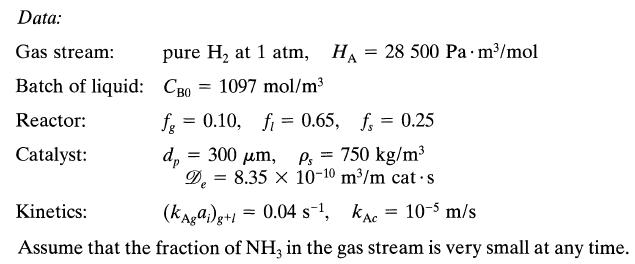
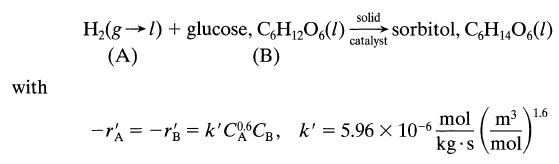
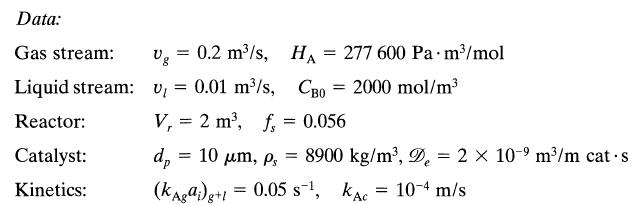
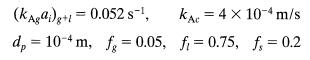
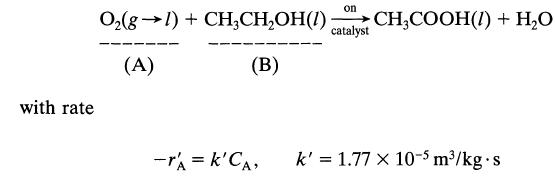

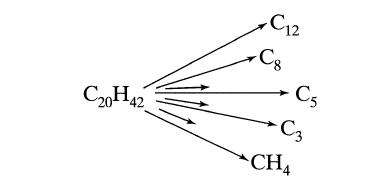
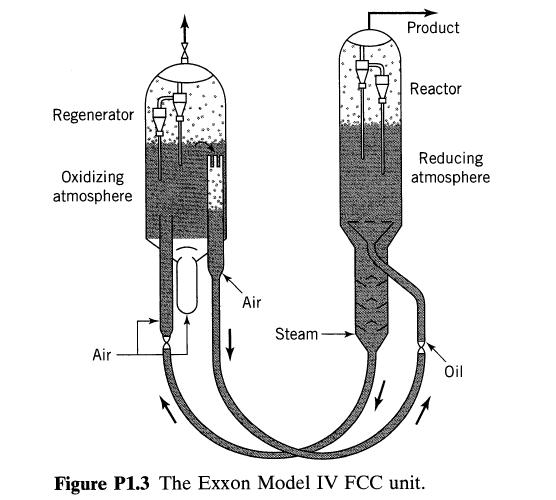
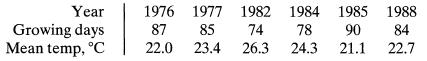
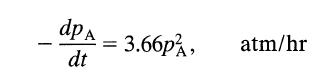
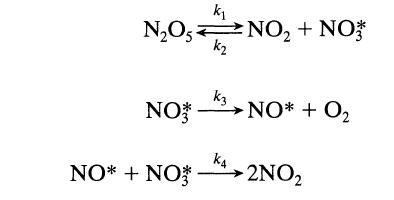
![+21/20 N,ON,+ k[NO] -N01+ k[NO]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1695/9/0/9/080651584d8b7d351695909078860.jpg)