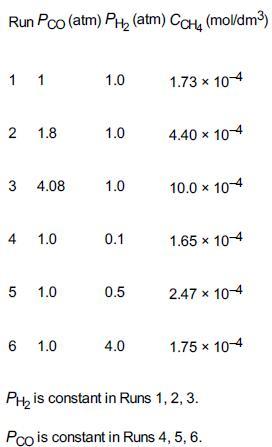
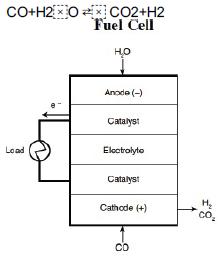
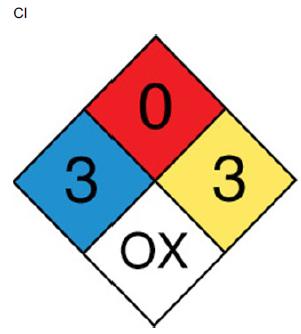
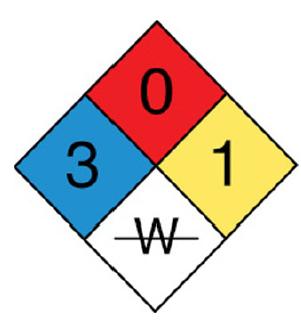
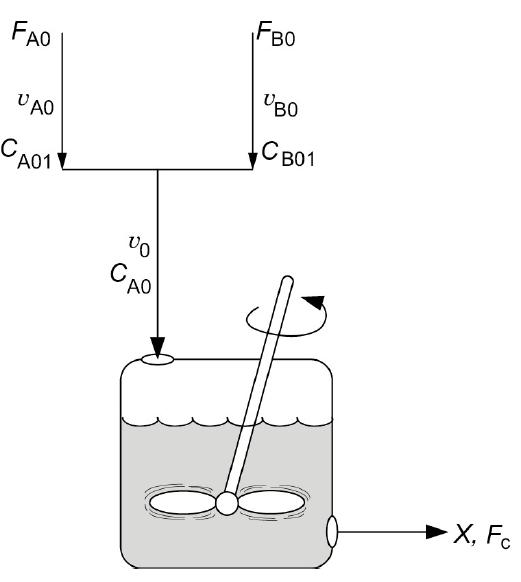
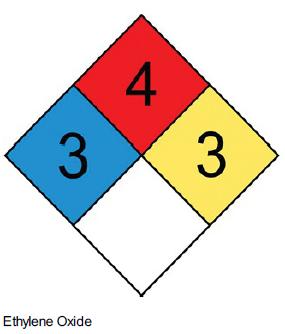
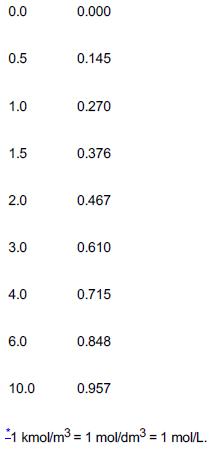
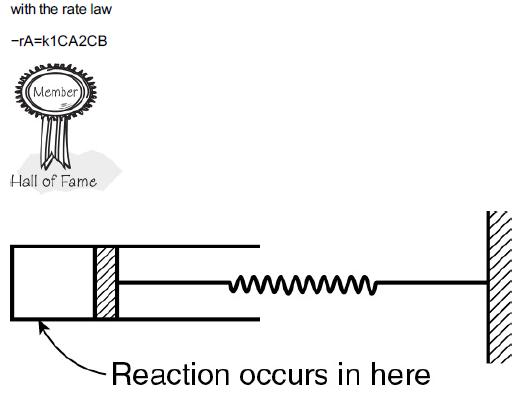
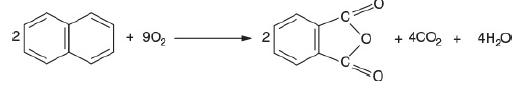
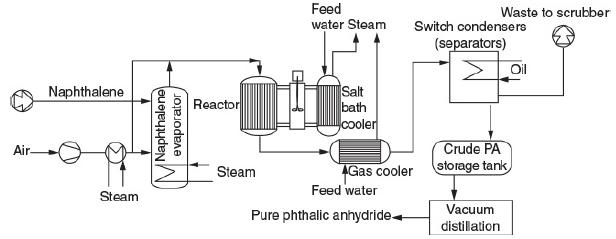
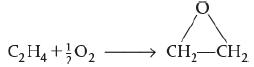
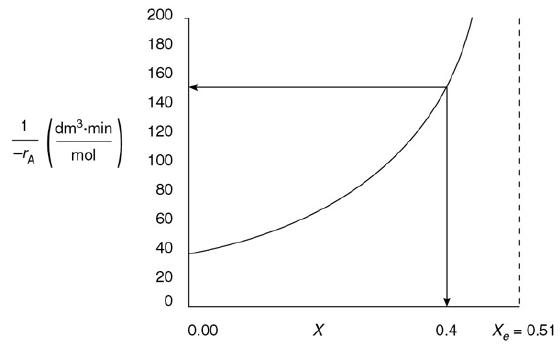
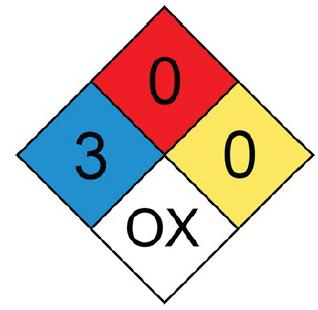
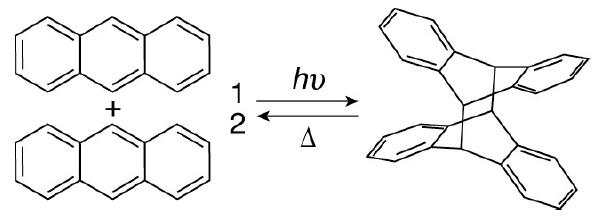
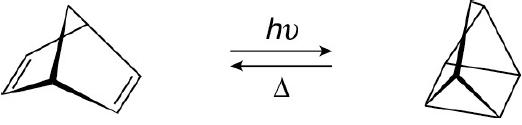
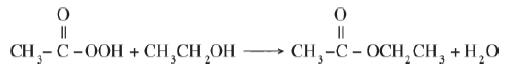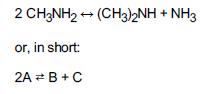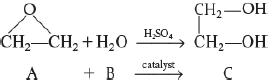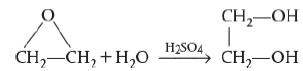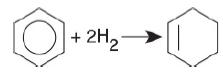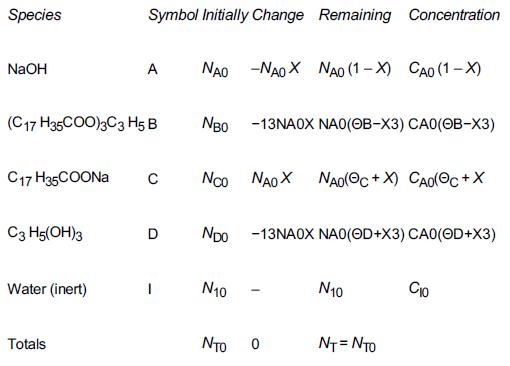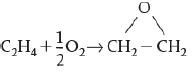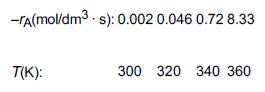![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


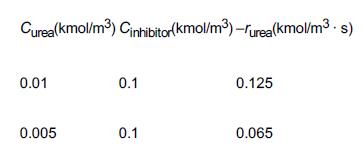






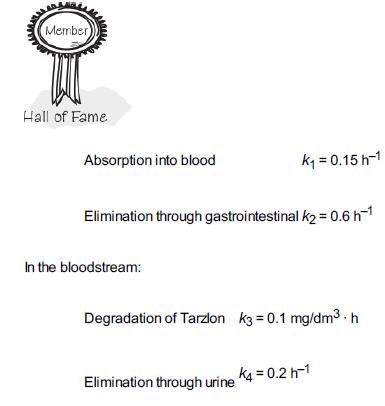
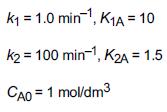
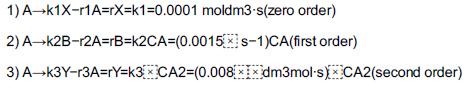
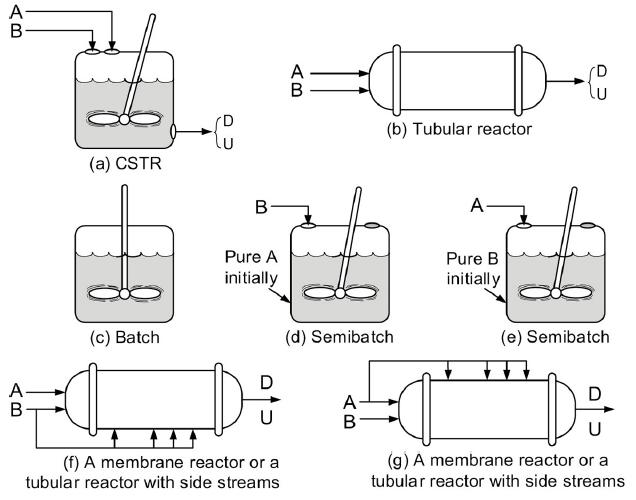
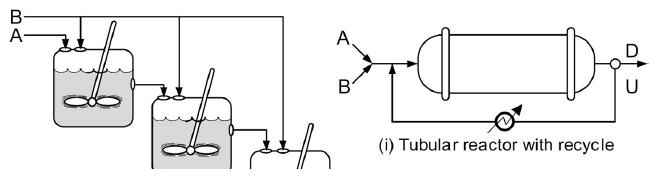
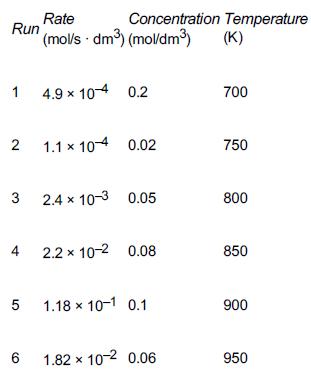
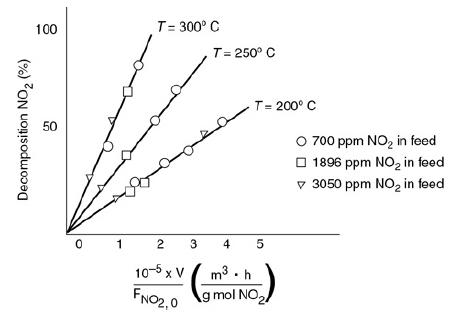
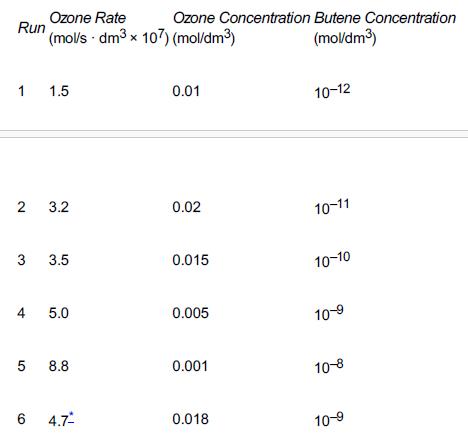






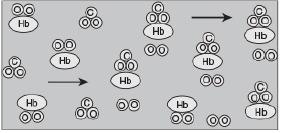
![t(min) 0 3 5 8 15 20 25 [% O] 21 15 12 10 5 3 2](https://s3.amazonaws.com/si.question.images/images/question_images/1697/1/7/1/9496528c9edebbf81697171949719.jpg)