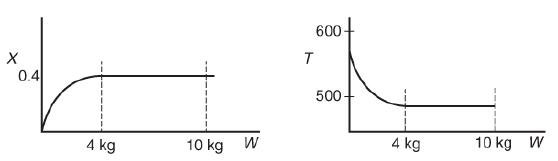
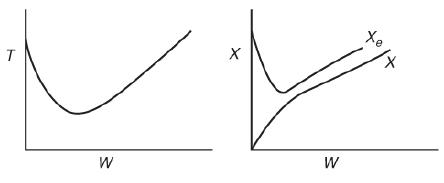
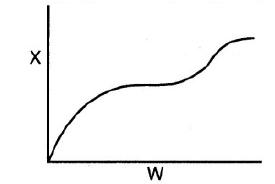
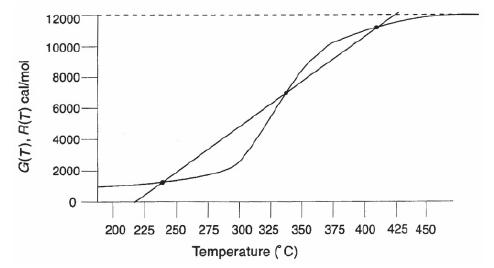
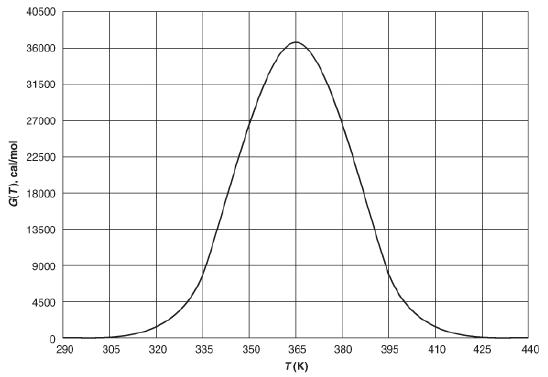
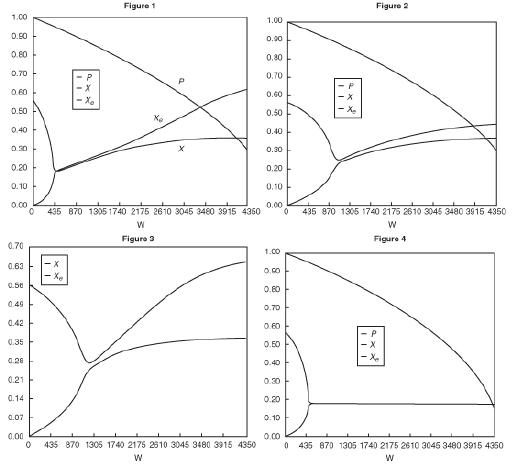
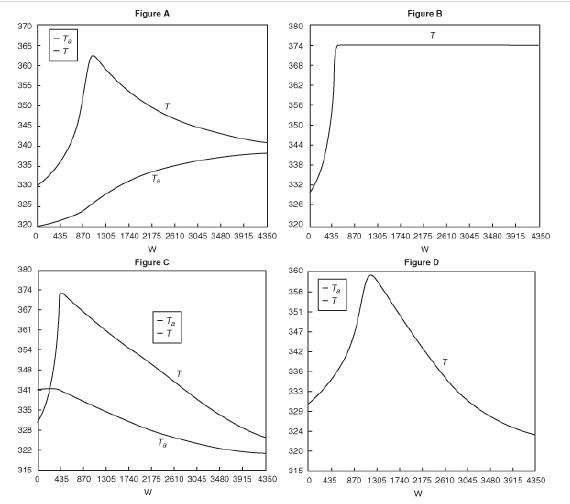
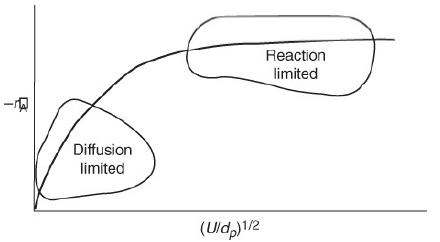
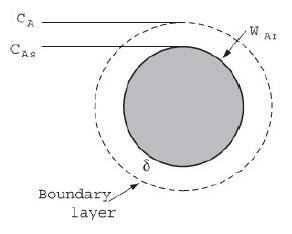
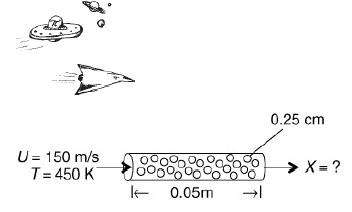
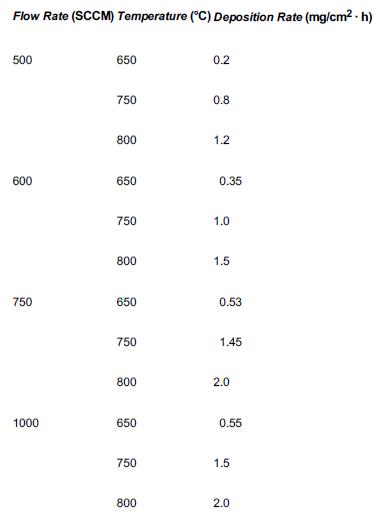
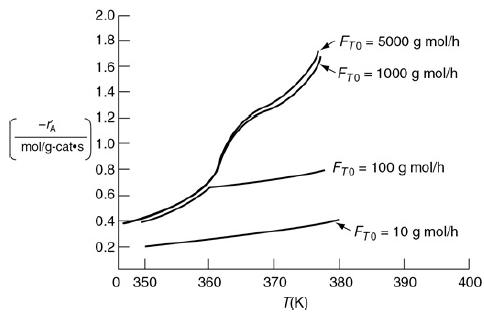
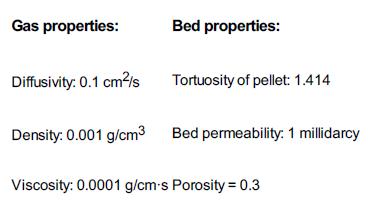
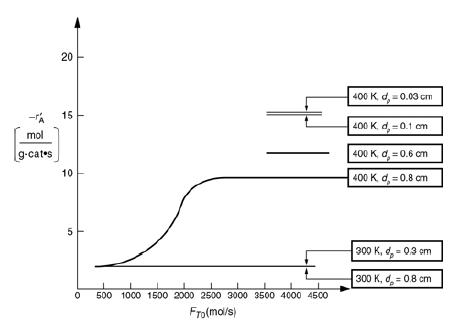
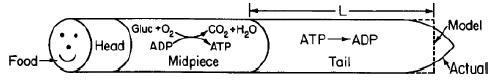
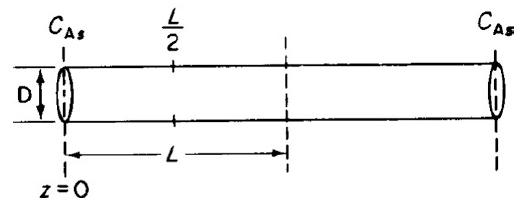
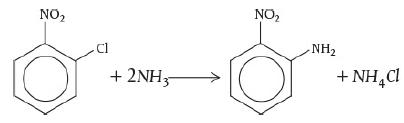
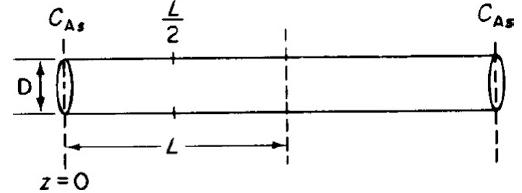
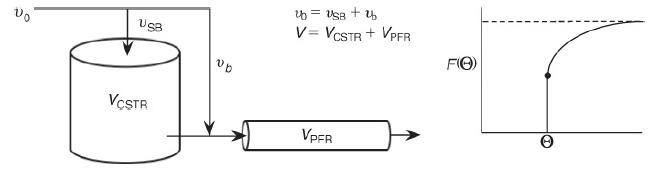
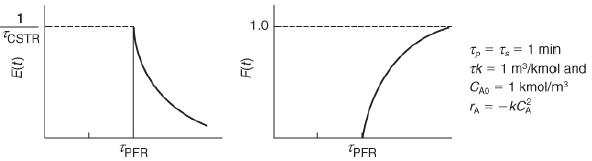
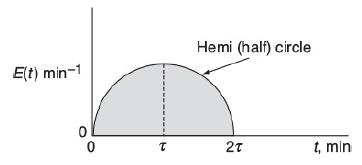
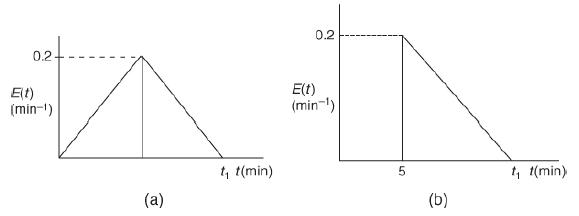
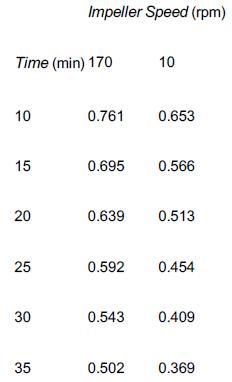
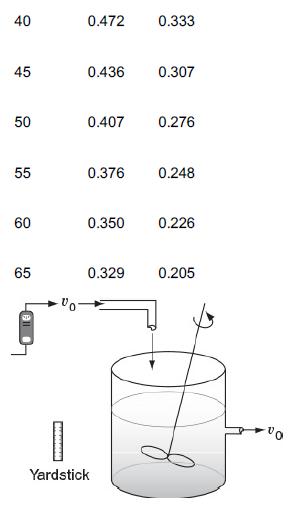
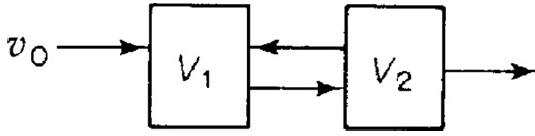
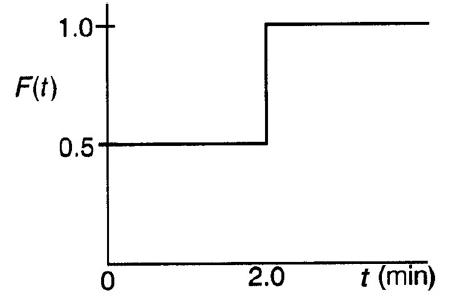
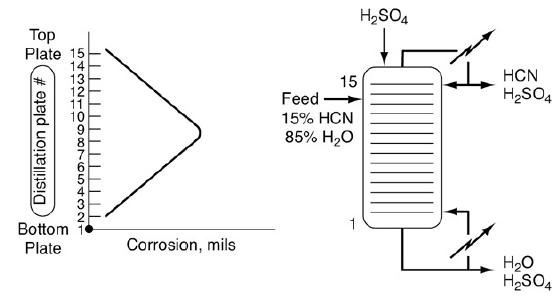
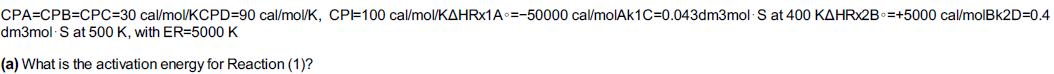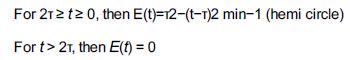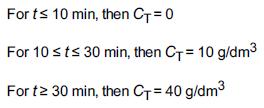![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()






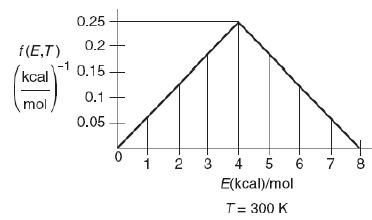
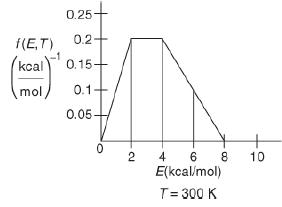
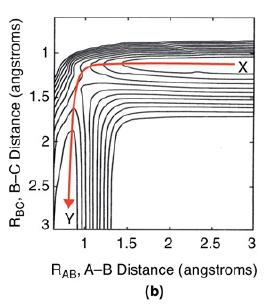
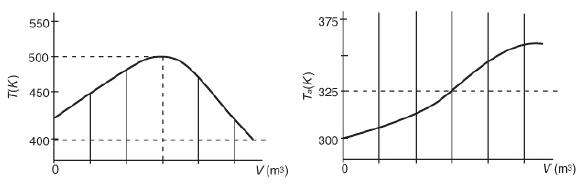
![f(E,T)dE=2(1KBT)3/2E1/2exp[-EkBT]dE(3-21) 0.9 m 0.81. 0.8 0.7 0.6 0.5 RET) 0.4 kcal 0.3 0.2 0.1 0 T - 300 K 0](https://s3.amazonaws.com/si.question.images/images/question_images/1697/0/0/5/62065264034435e71697005618607.jpg)
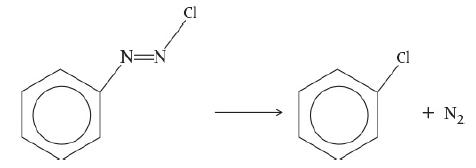
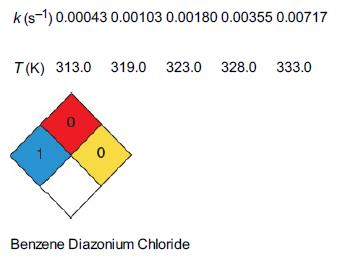
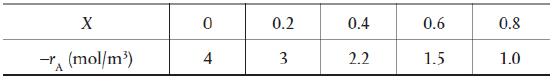
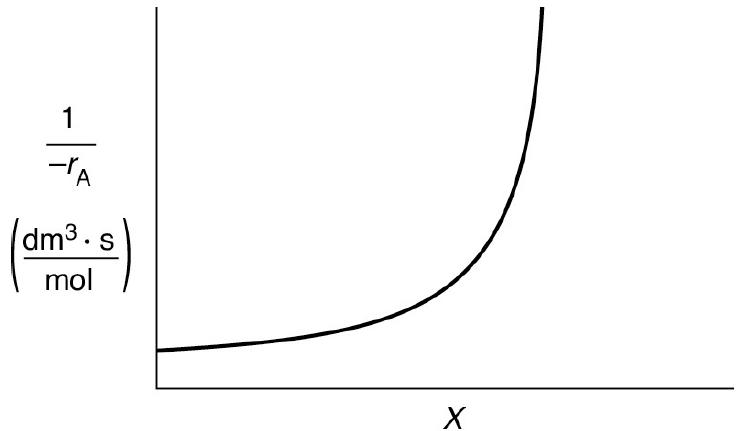
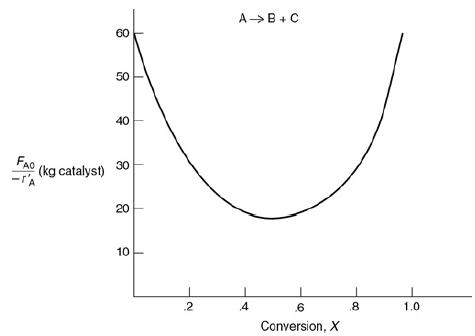


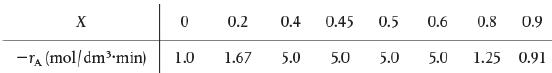
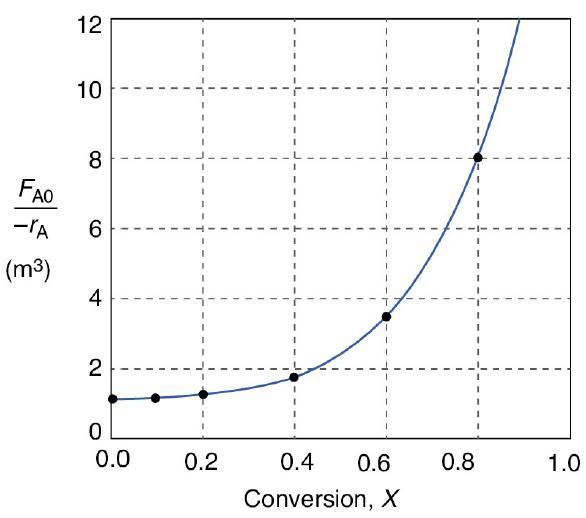
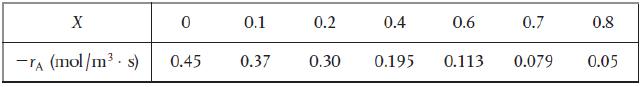
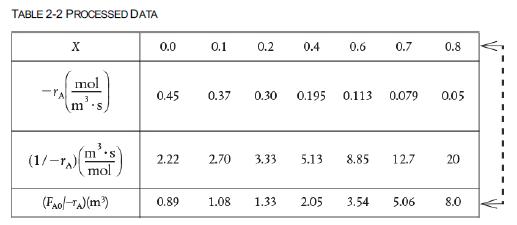
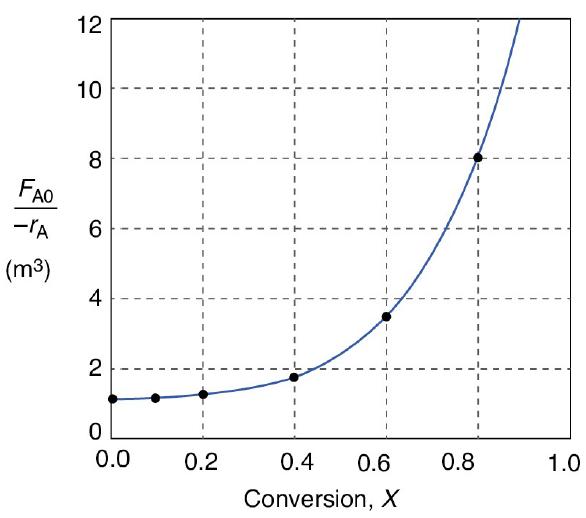

![HAO(273K)* =-20 kcal/mol,HBO(273x K)-15 kcal/mol,HCo(273K) =-41[x] kcal/mol CPC=30x cal/mol](https://s3.amazonaws.com/si.question.images/images/question_images/1697/6/1/0/505652f7b09e63471697610504453.jpg)