![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


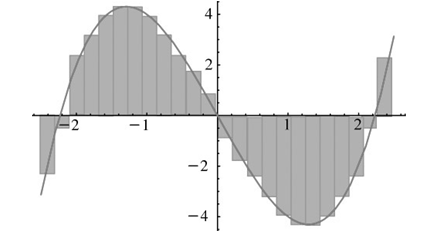
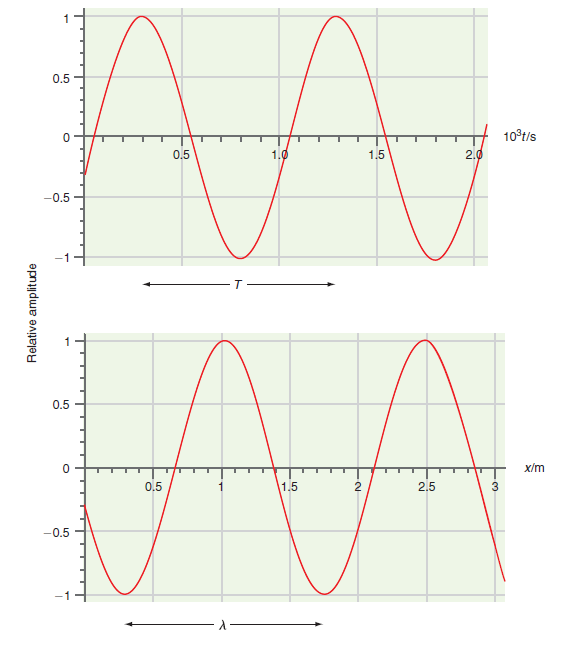
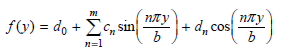
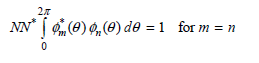
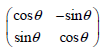
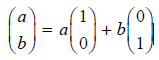
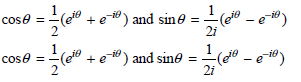
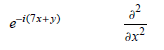
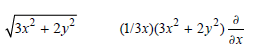
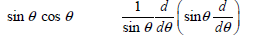

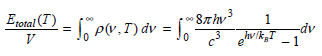
![– 1)] dx = n*/15.](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/8/0/5/5475ae2afab31ddd1524805531880.jpg)