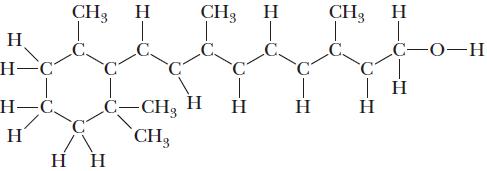
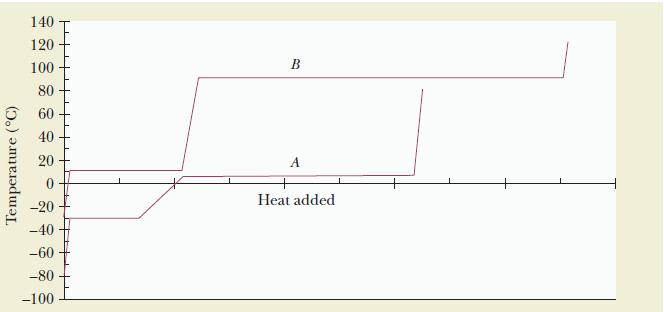
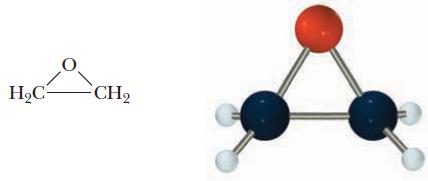
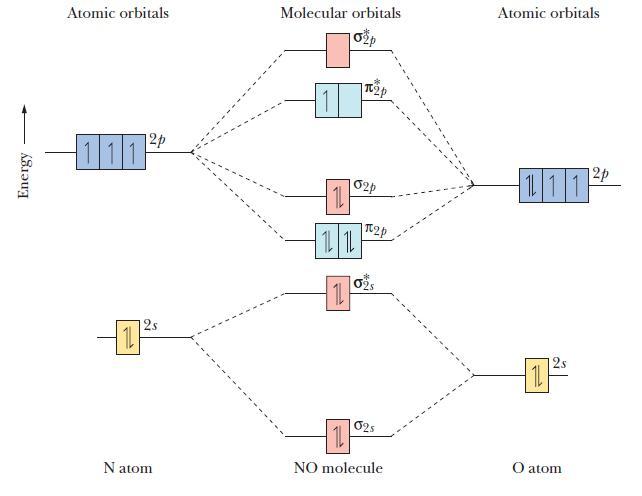
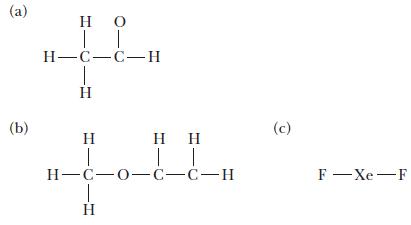
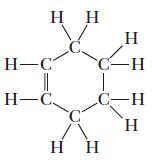
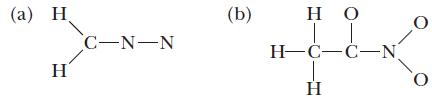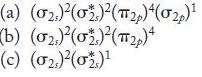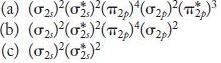![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


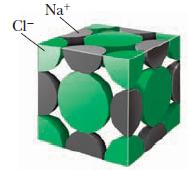
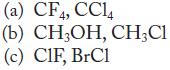
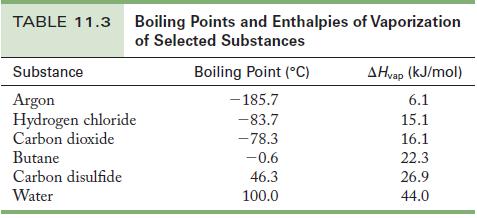
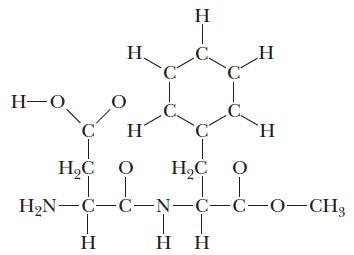

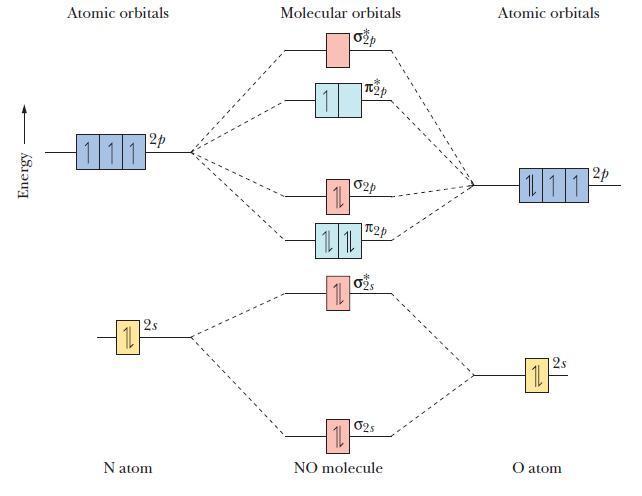
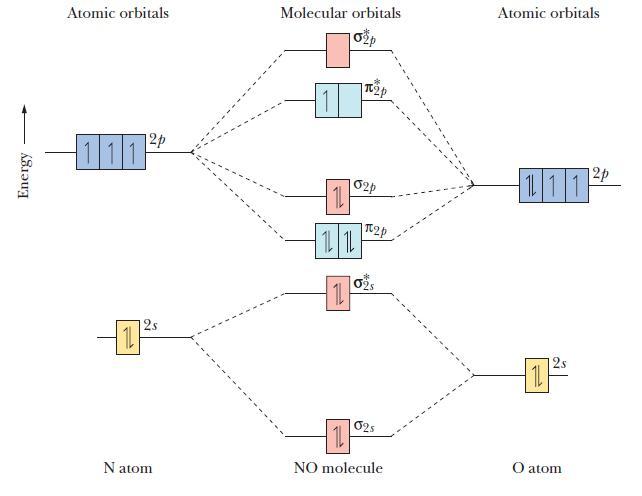
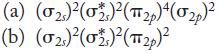
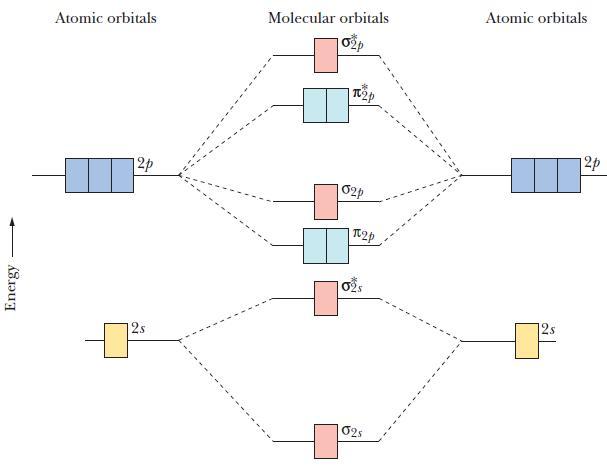
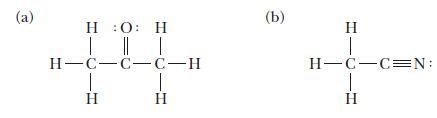

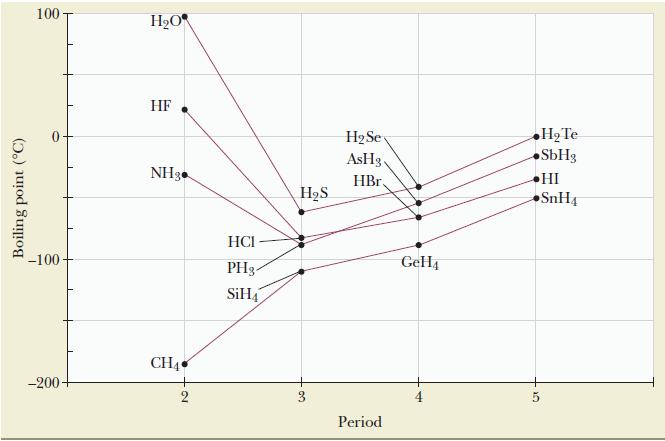
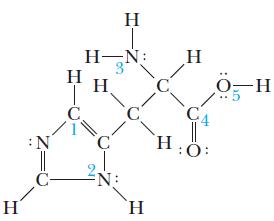
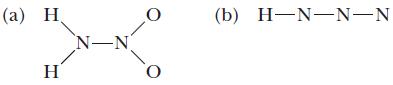
![(a) [0- C-N] (b) 0 _O-N-O_](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/1/9/7/1186593fbfe13fb71704197117784.jpg)