![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


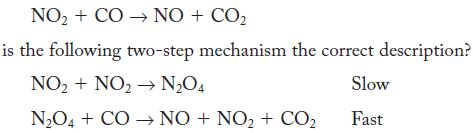
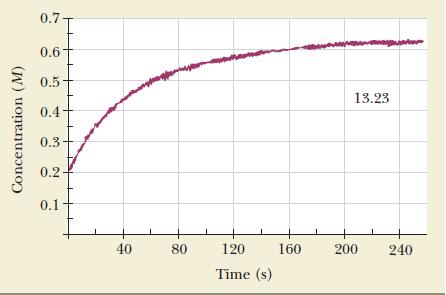
![NO + CO NO + CO The rate law, found by experiment, is second order: rate = k[NO]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/4/4/95265963d78871df1704344952027.jpg)
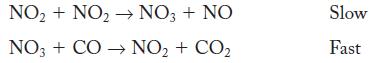
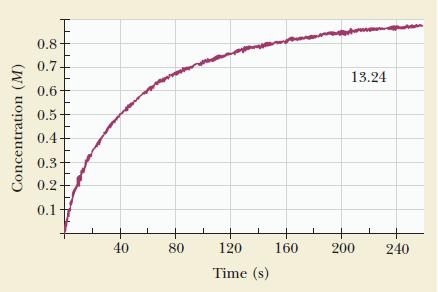
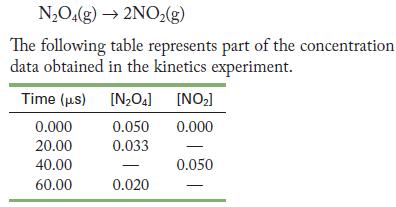
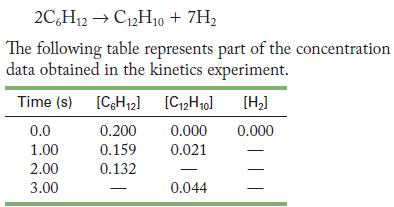
![(a) rate = k[NO][NO] (b) rate = k[O]/2[C1] (c) rate k[HCIO][OH-] =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/64165965f69d628c1704353641585.jpg)
![2NO(g) + 2H(g) N(g) + 2HO(g) Experiment 1 2345 Initial Concentration (M) [NO] 0.10 0.10 0.10 0.20 0.30 [H]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/70365965fa70a8711704353702734.jpg)
![k[O3][NO] (a) rate = (b) rate= [NO]/ [C1] (c) rate = k[CH3Br][OH-]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/65565965f772eefc1704353654897.jpg)
![NO(g) + HO(g) 2NO(g) + H(g) Experiment 1 23 Initial Concentration (M) [NO] [HO] 0.12 0.10 0.12 0.20 0.25](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/74465965fd0eb79d1704353744583.jpg)
![HI(g) + CH;I(g) CH.(g) + L(g) Experiment 1 123 Initial Concentration (M) [HI] 0.053 0.106 0.106 [CH5l] 0.23](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/76165965fe13e4701704353760938.jpg)
![NO(g) + O3(g) NO3(g) + O(g) Experiment 1 2 3 4 Initial Concentration (M) [NO] 2.0 x 10-6 3.0 x 10-6 4.0 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/77765965ff16e9aa1704353777138.jpg)
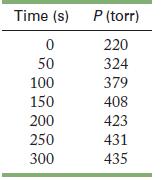
![PH3(g) + BH.(g) PH3BH3(g) + BH3(g) Experiment 1 7234 Initial Concentration (torr) [BH6] 1.2 1.2 1.2 3.0](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/3/8946596606606bc41704353893552.jpg)
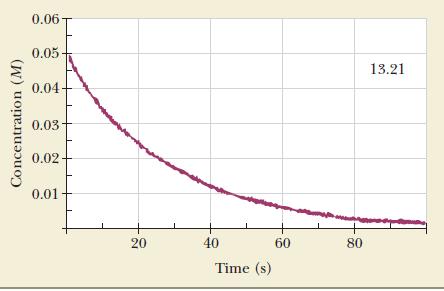
![Time (s) [Reactant] (M) 0.250 0 1 0.216 2 0.182 0.148 0.114 0.080 3 4 5](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/4/018659660e2f164f1704354018702.jpg)
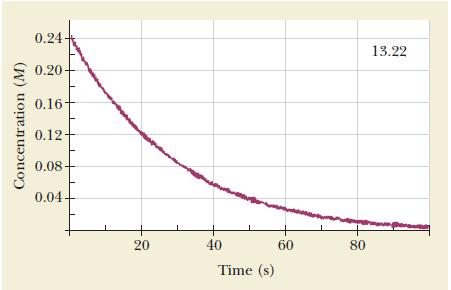
![Time (s) 0 2 15 [Reactant] (M) 0.0451 0.0421 0.0376 0.0316 0.0226](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/4/041659660f9ad7751704354040910.jpg)
![Time (s) 0.001 0.002 0.003 0.004 0.005 [Reactant] (M) 0.220 0.140 0.080 0.050 0.030](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/4/1066596613a2b0b41704354105848.jpg)
![Time (s) 0 10 20 50 70 [Reactant] (M) 0.0350 0.0223 0.0142 0.0037 0.0015](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/4/133659661557b7371704354133147.jpg)
![NOCI(g) NO(g) +- Ch(g) Time (s) 0 30 60 100 200 300 400 [NOCI] (M) 0.100 0.064 0.047 0.035 0.021 0.015 0.012](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/4/1516596616749a601704354150888.jpg)
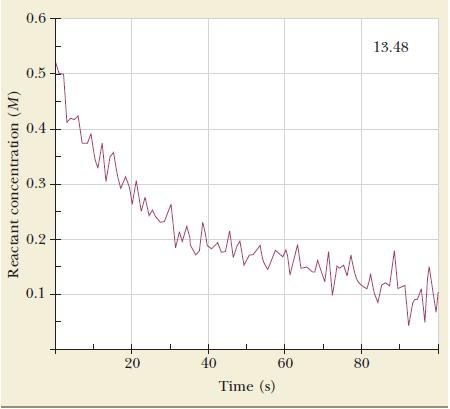

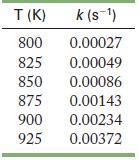
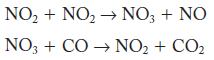
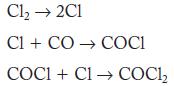
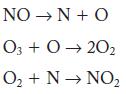
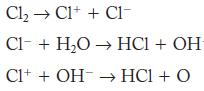
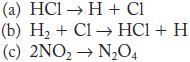
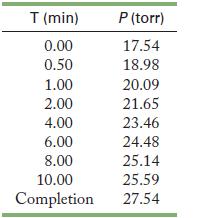
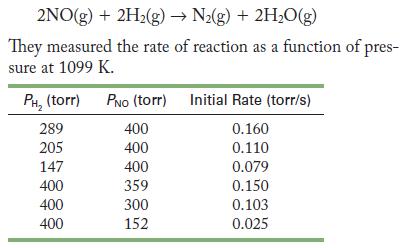
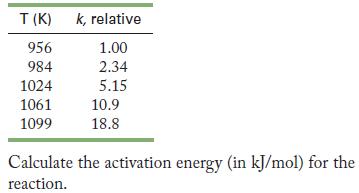
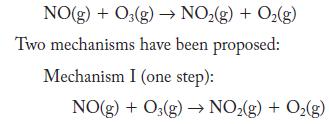
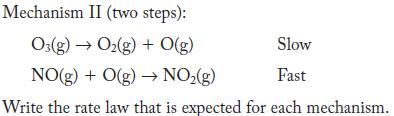
![2NO(g) + Cl(g) 2NOC1(g) rate = k[NO][C1] Evaluate the following proposed mechanism to determine whether it](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/143659665475f29b1704355142932.jpg)
![2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/041659664e176f1c1704355041072.jpg)
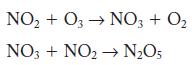
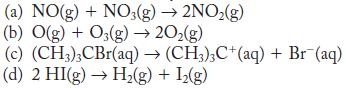
![2NO2+O3 NO5 + O rate = k[NO][03] k (a) 2NO NO4 k NO4 + 03 NO5 + O (b) NO + 03 NO3 + O NO3 + NO NO5 Fast,](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/17165966563b233b1704355171135.jpg)
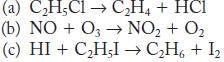
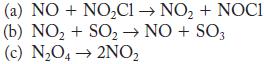
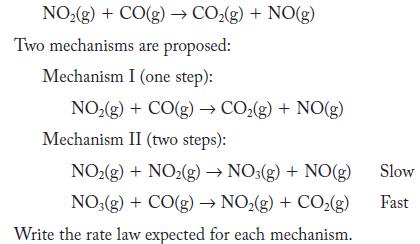
![Time (s)[H2O2] (M) 0 10 20 30 40 50 60 0.0334 0.0300 0.0283 0.0249 0.0198 0.0164 0.0130](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/44865966678b27851704355448171.jpg)
![Time (s) 0 900 1800 2700 3600 7200 10800 18000 [pTSA], M 0.100 0.0863 0.0752 0.064 0.0568 0.0387 0.0297 0.0196](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/414659666561eb001704355413691.jpg)
![CO + Cl COC1 Experiment 123 Initial Concentration (M) [CO] 0.053 0.106 0.106 [C] 0.23 0.23 0.46 Initial Rate](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/475659666933ac681704355474758.jpg)
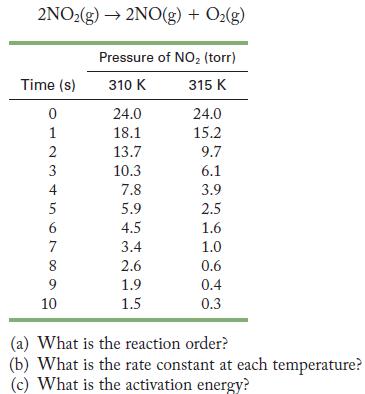

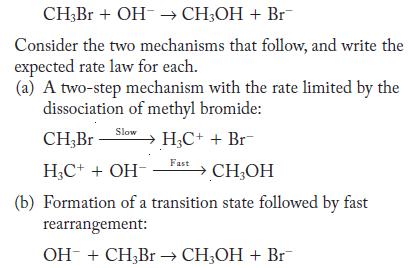
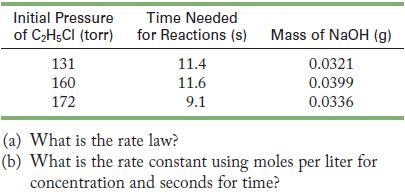
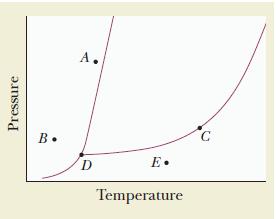

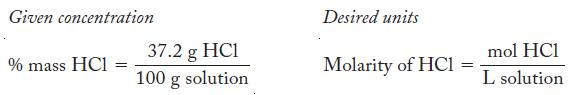
![(a) Carbon tetrachloride [CC14()] in liquid water or hexane [C6H4(e)] (b) Urea [CO(NH)2(s)] in water or](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/2/6/4/668659503dcc39b71704264668454.jpg)