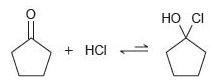
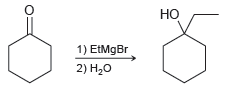
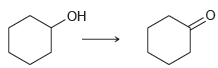
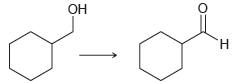
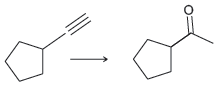
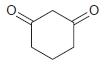
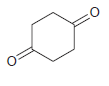
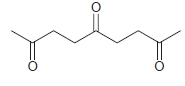
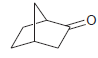
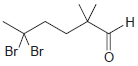
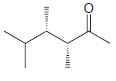
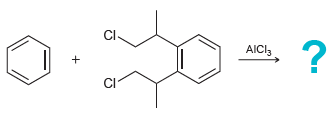
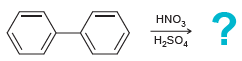
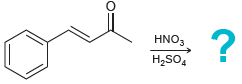
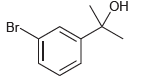
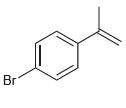
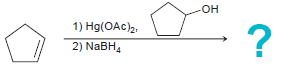
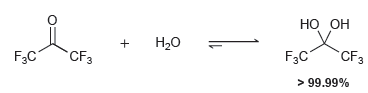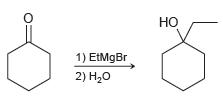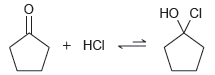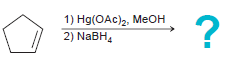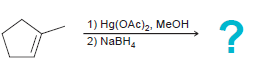![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


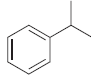
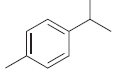
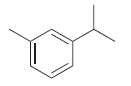
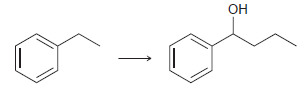
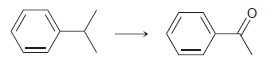
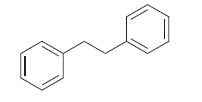
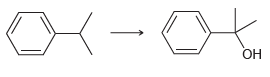
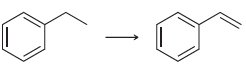
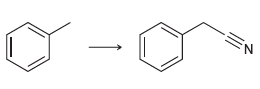
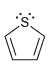
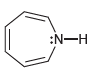
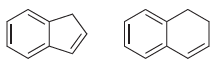
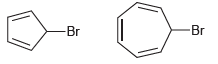
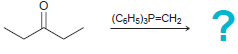

![[Н'] н но ELOH](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/5/0/5175ae05215c43481524650498574.jpg)
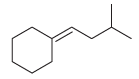
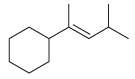
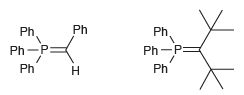
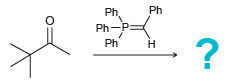
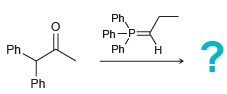
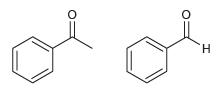
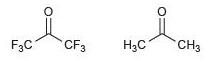
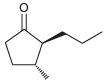
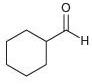

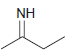
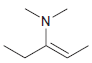
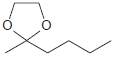
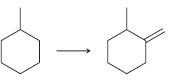
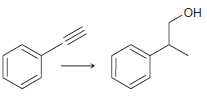
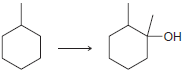
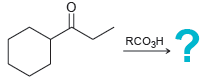
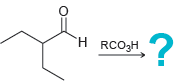
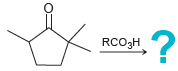
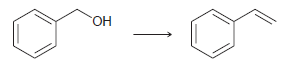
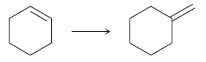
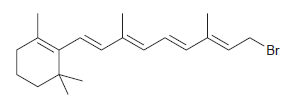
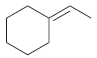
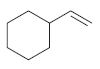
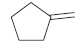
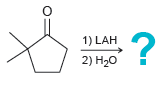
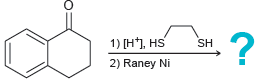
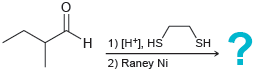
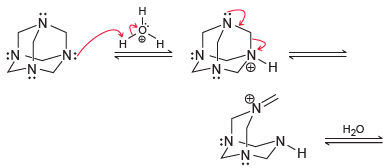
![[H2SO4] N- -ОН но. Н-N.](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/8/2695ae0494d8649b1524648247951.jpg)
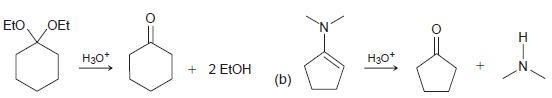
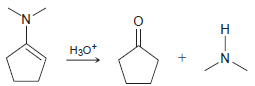
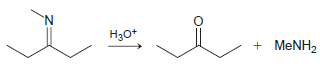
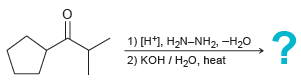
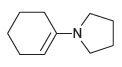
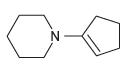
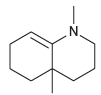
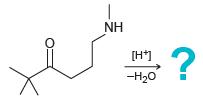
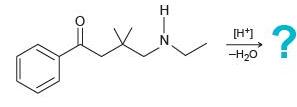
![[H*] N-H -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/9555ae03c5b36c131524644939279.jpg)
![Н :? [н] -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/9555ae03c5b76be61524644939607.jpg)
![[H2SO4] Et,NH 'N' -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/9115ae03c2f84a321524644891327.jpg)
![[H2SO4] Me-NH -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/9125ae03c301a17c1524644891722.jpg)
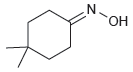
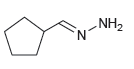
![[н1] но-NH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/7585ae03b964fc9d1524644739370.jpg)
![[H*] H2N-NH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/7585ae03b96935ec1524644739673.jpg)
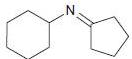
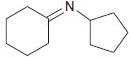
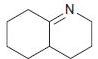
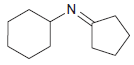
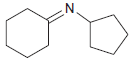
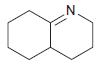
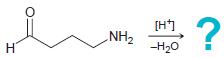
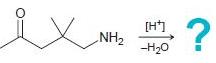
![[H*] -Н2о NH2 Н](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/4455ae03a5d09ac71524644416433.jpg)
![[H] „NH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/4455ae03a5db27d61524644416757.jpg)
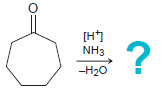
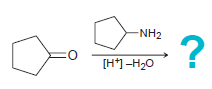
![[TSOH] MENH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/2195fc4c5d334dde1606731218633.jpg)
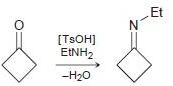
![[TSOH] MENH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/0935ae038fd72d991524644076441.jpg)
![Et [TSOH] EENH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/4/0935ae038fdb5df91524644076744.jpg)
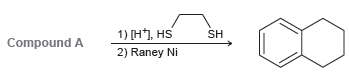
![Но, Compound A [H*]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/9355ae0385f449ad1524643914277.jpg)
![Но, [H2SO4] ОН](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/8945ae038366890d1524643865163.jpg)
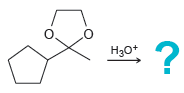
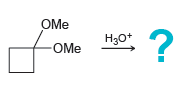
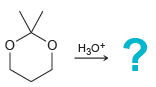
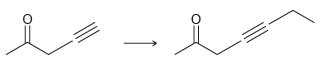

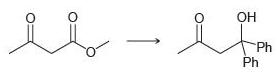
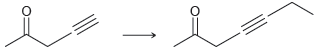
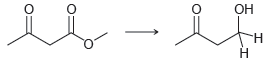
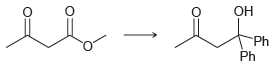
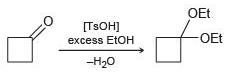
![[H2SO4] excess MeOH -? -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/5975ae0370d7a4411524643575525.jpg)
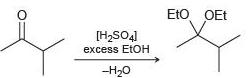
![но [H,SO4] он -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/5975ae0370db39921524643575861.jpg)
![HO HO- [H2SO4] -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/3595fc4c65f2b52c1606731358604.jpg)
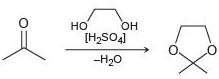
![Но ОН [H,SO4] -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/4865ae0369e83c8e1524643464957.jpg)
![но [H,SO4] он -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/6/4/3/4865ae0369ee18121524643465261.jpg)
![Meo, OMe [H,SO4] excess MeOH -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/5905fc4c746e5bb91606731590218.jpg)