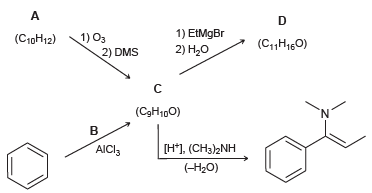
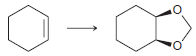
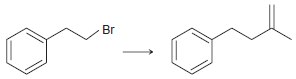
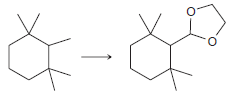
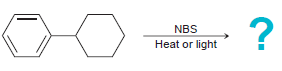
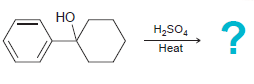
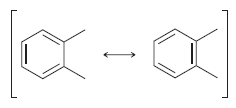
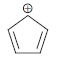
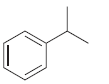
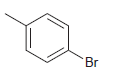
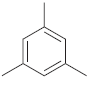
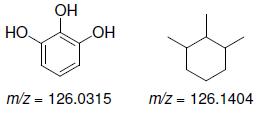
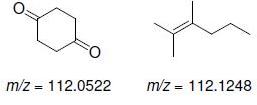
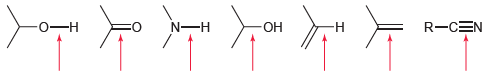
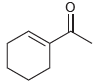
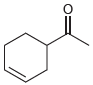
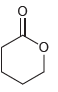
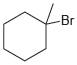
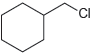
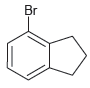
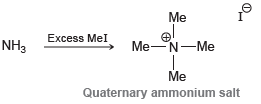
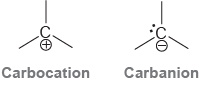
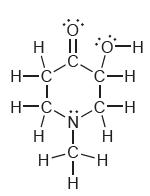
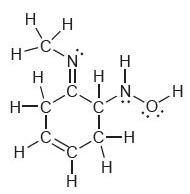
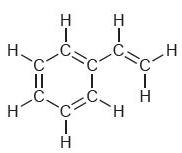
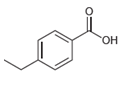
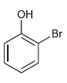
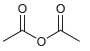
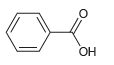
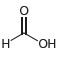
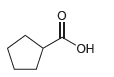
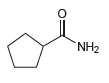
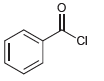
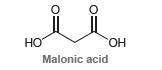
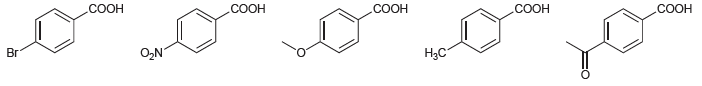
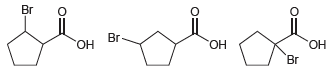
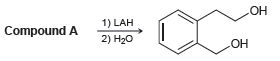
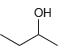
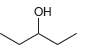
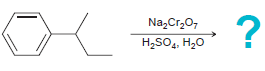![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


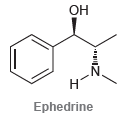
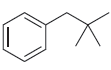
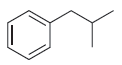
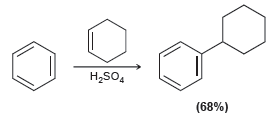
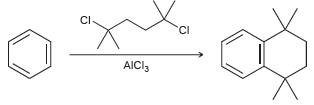
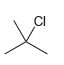
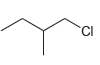
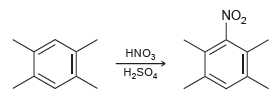
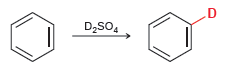
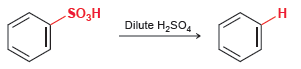
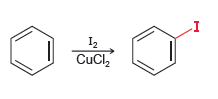
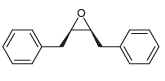
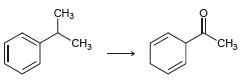
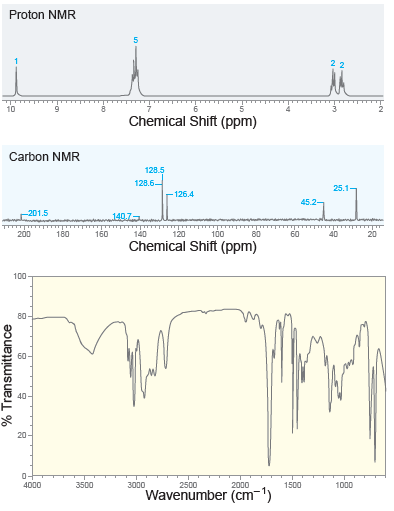
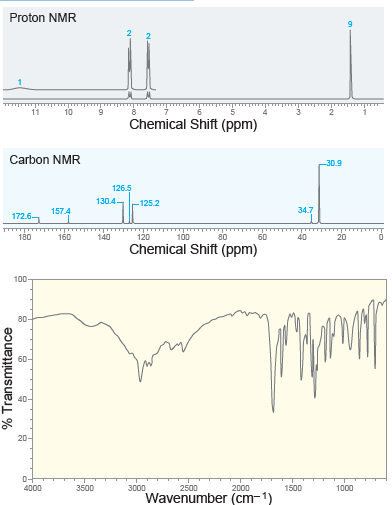
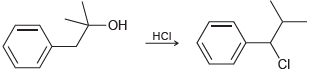
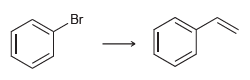
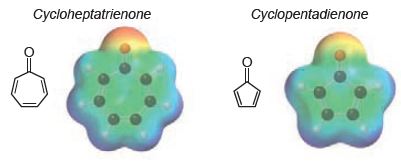
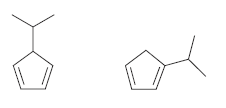
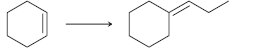

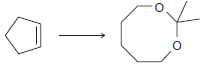
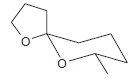
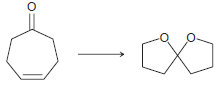
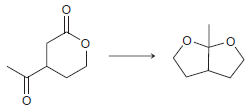
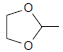
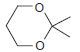
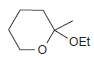
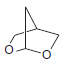
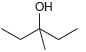
![CHз NH2 [H*] (-H20)](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/7/1/8/6025ae15c0a95d891524718586667.jpg)
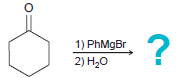
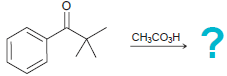
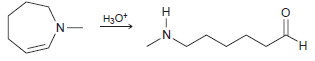
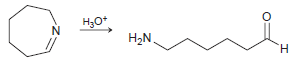
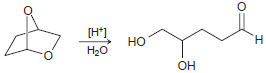
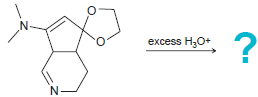
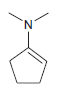
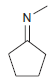
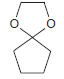
![он но [Н0] Н Н Glutaraldehyde](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/7/1/8/1725ae15a5cce2c91524718157210.jpg)
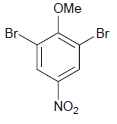
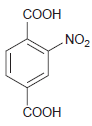
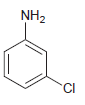
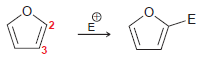
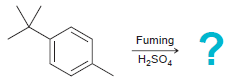
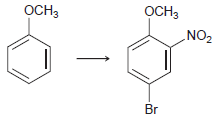
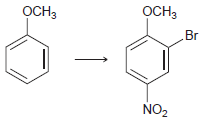
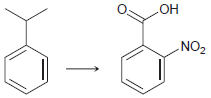
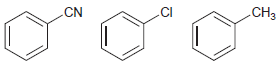
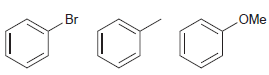
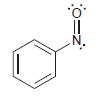
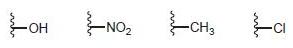
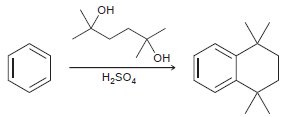
![-NH2 [H,SO] NH2 [-H,0]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/7/1/8/8135ae15cddb5a241524718798339.jpg)
![1) Br2 н Mg A. A FeBr3 H. 2) H20 PCC но Он [н], -Н-0 ш](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/7/1/9/7885ae160ace04581524719773593.jpg)
![но, А Н,о* H,Cro, в н] NH,NH, (-Н-0) КОН/Нао D heat](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/7/1/9/7745ae1609e581301524719758915.jpg)