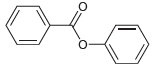
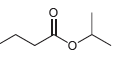
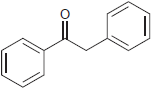
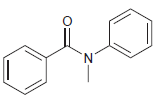
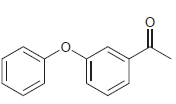
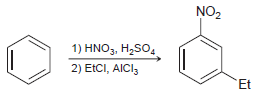
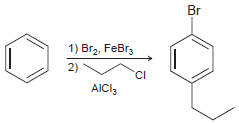
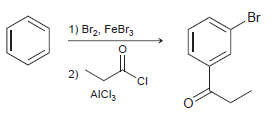
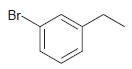
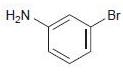
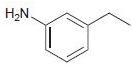
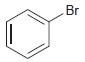
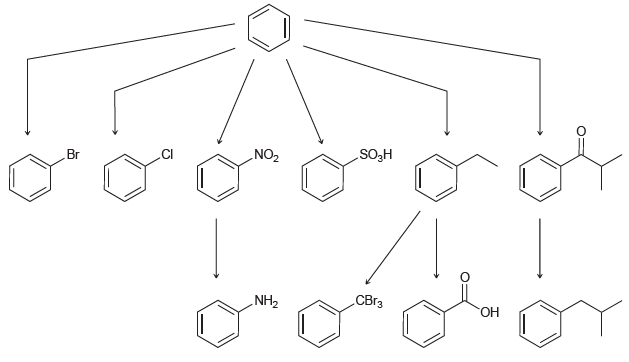
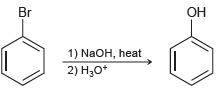
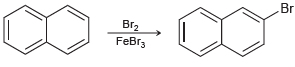
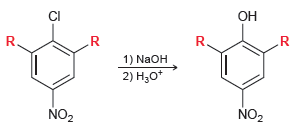
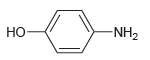
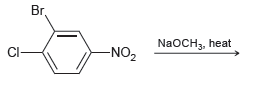
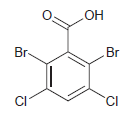
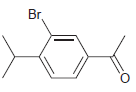
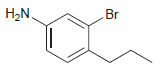
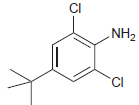
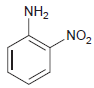
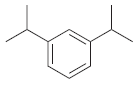
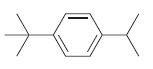
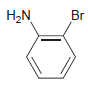
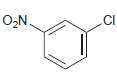
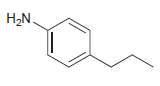
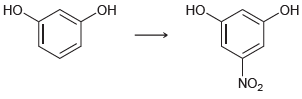
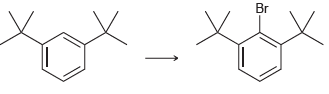
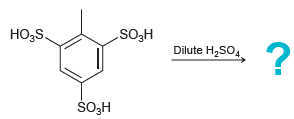
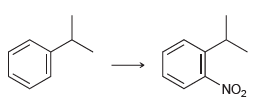
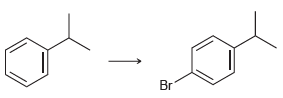
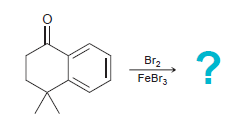
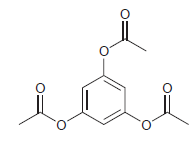
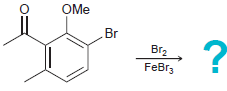
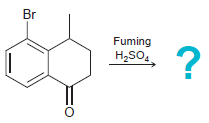
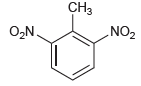
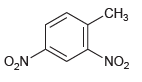
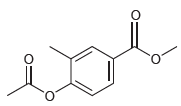
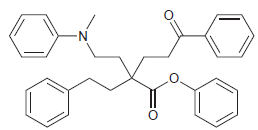
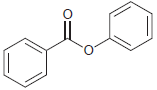
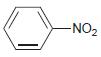
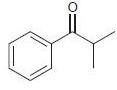
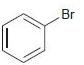
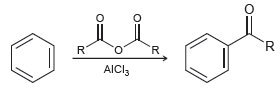
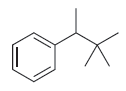
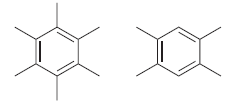
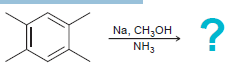
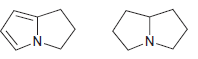
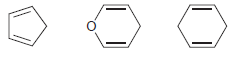
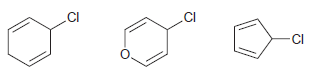
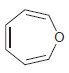
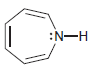
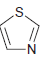
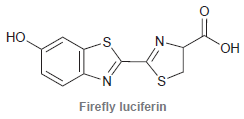
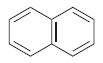
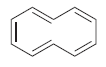
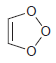
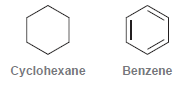
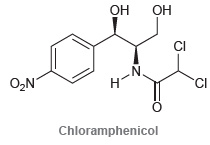
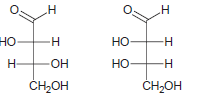
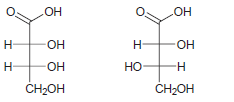
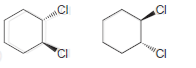
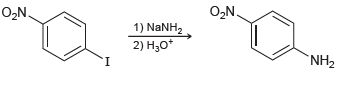![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


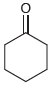
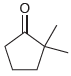
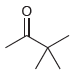
![[H,SO,] Но НО R.](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/8/1/0/4105ae2c2aaab9221524810398303.jpg)
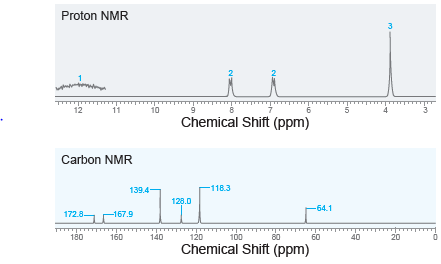
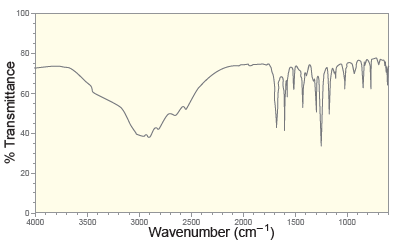
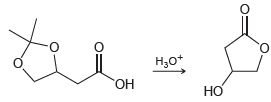
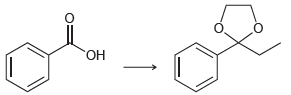
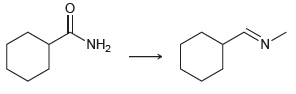
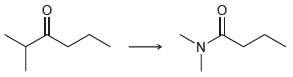
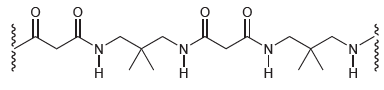
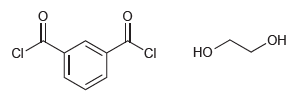
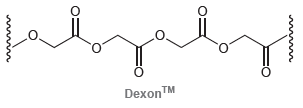
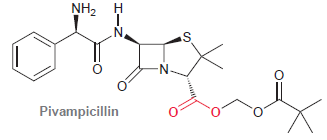
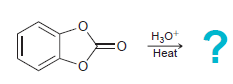
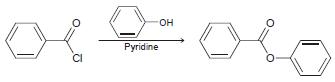
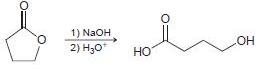
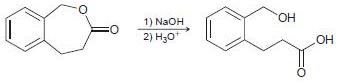
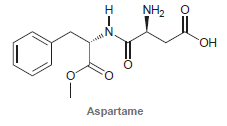
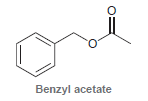
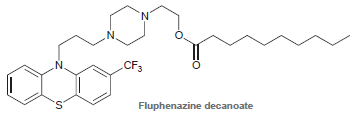
![[н]. H20 ОН Он он](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/8/0/9/8925ae2c0a490b4a1524809880332.jpg)
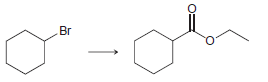
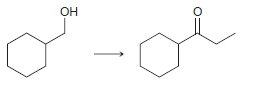
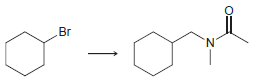
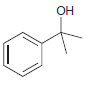
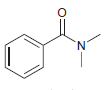
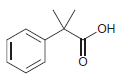
![ОН Na,Cr,0, H2SO4, H20 [H'] A EtOН soch 1) LIAI(OR)H `2) H20 xS NH3](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1524/8/0/9/5785ae2bf6a64bb51524809566185.jpg)