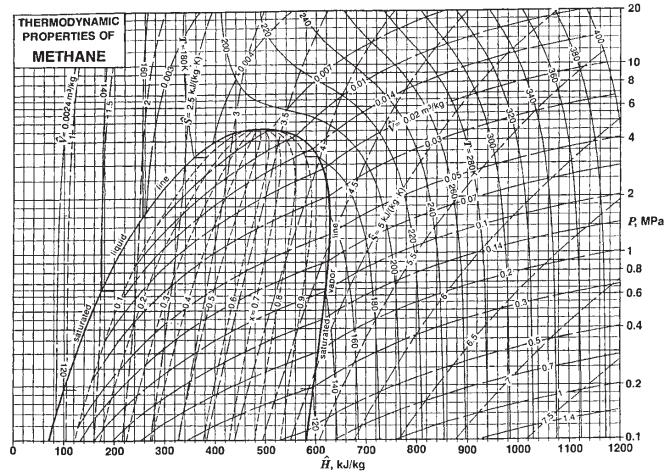
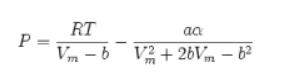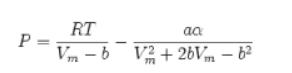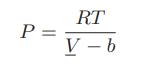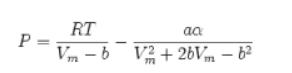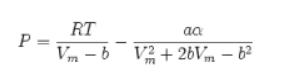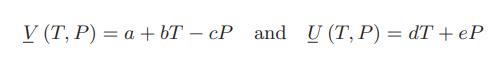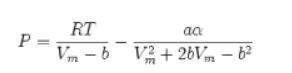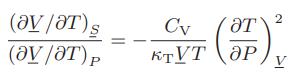![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


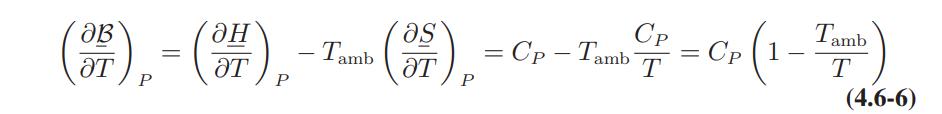
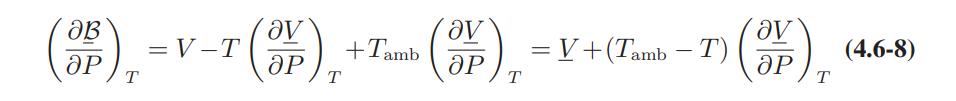
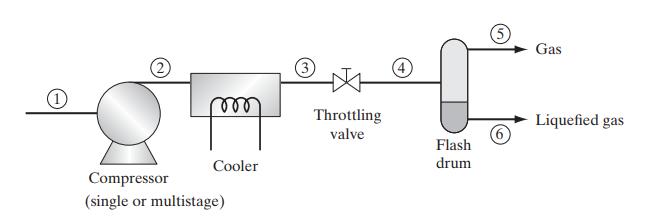
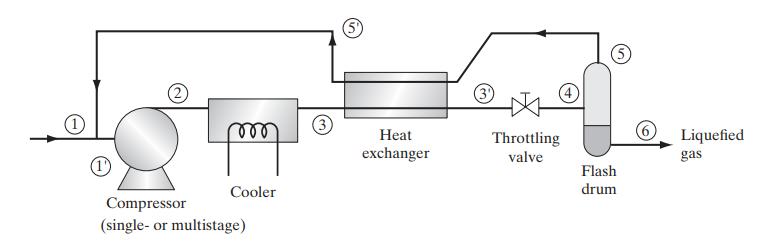
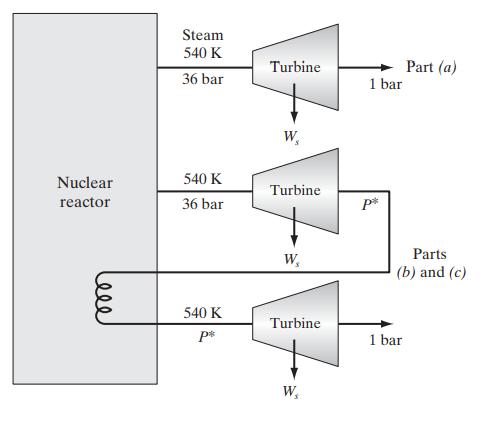

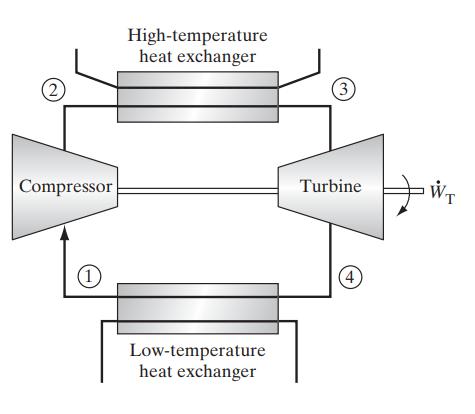
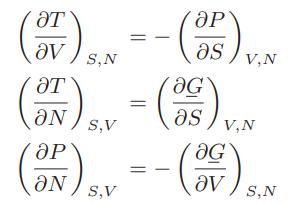
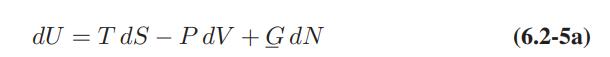
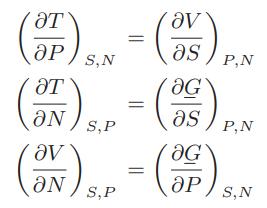
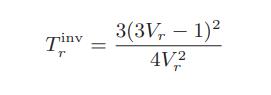
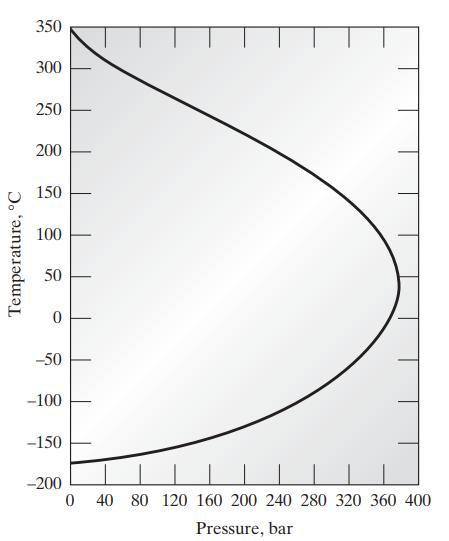
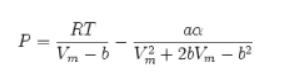
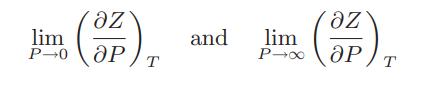
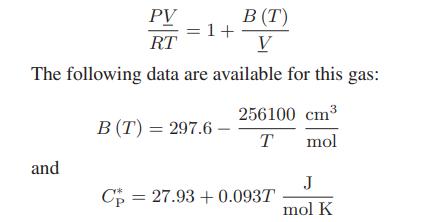
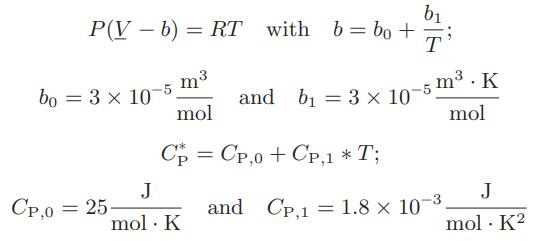
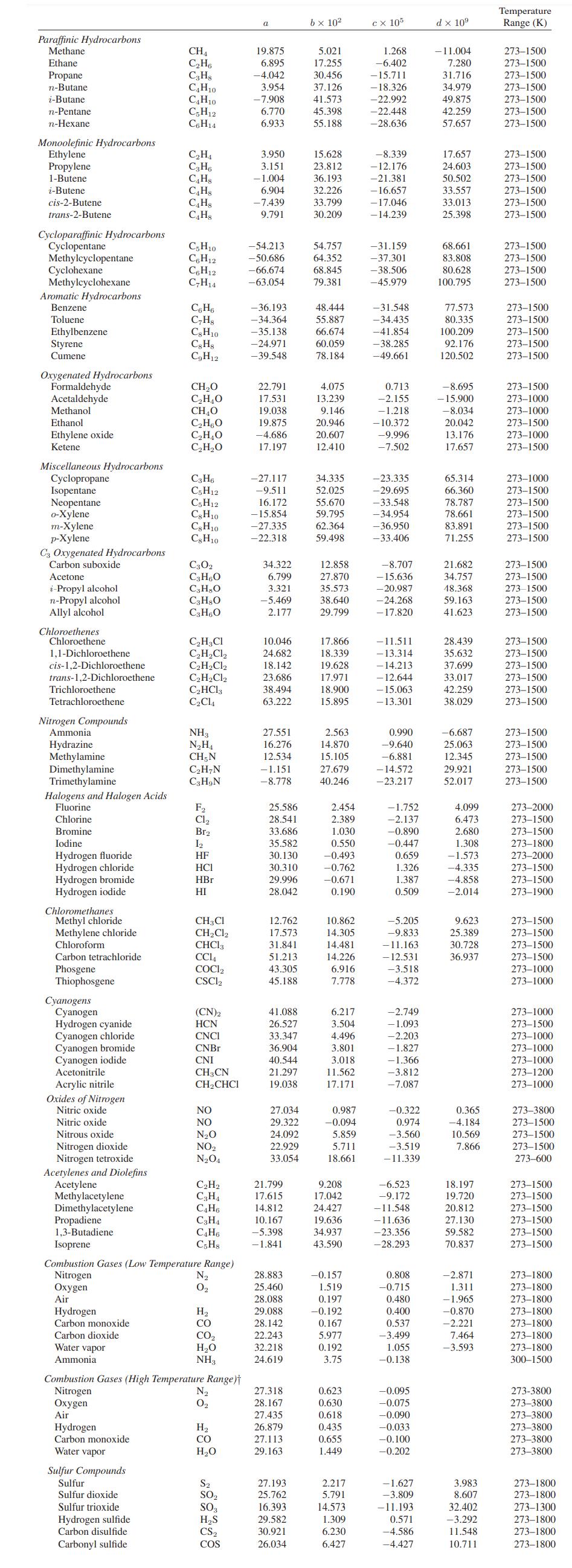
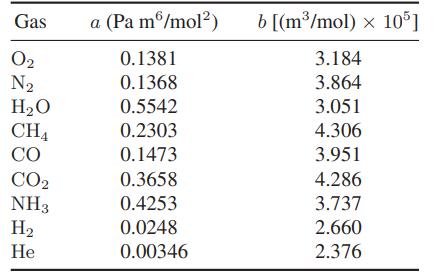
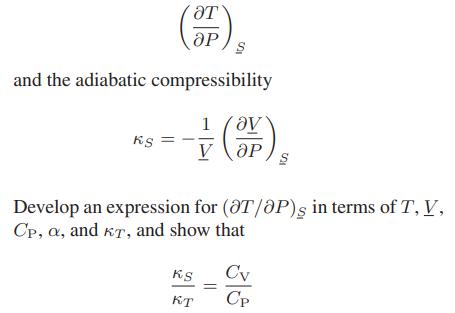
![Pr,Tr S(T,P)-SIG (T, P) = - R R S Pr=0,Tr (OZ.) P.] Z-1 Tr + Pr Pr dP (6.6-11)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/3/9/1636553035b418db1699939160220.jpg)
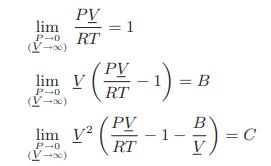
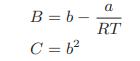
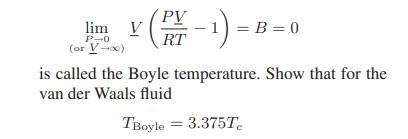
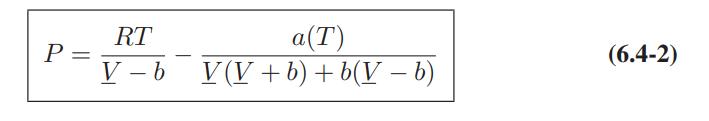
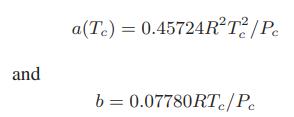
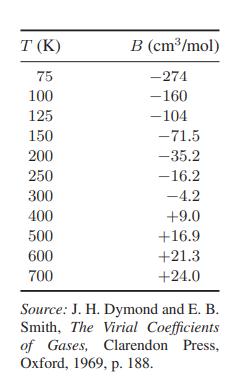
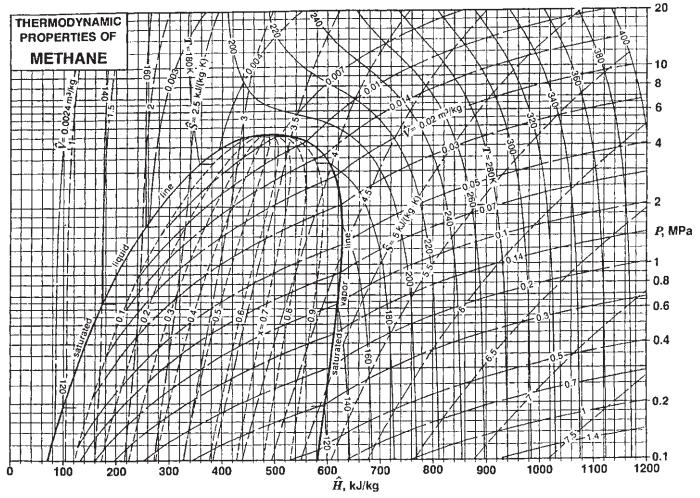
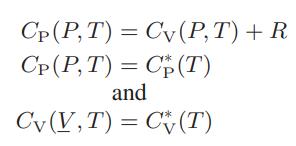
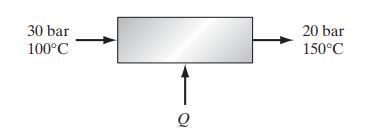
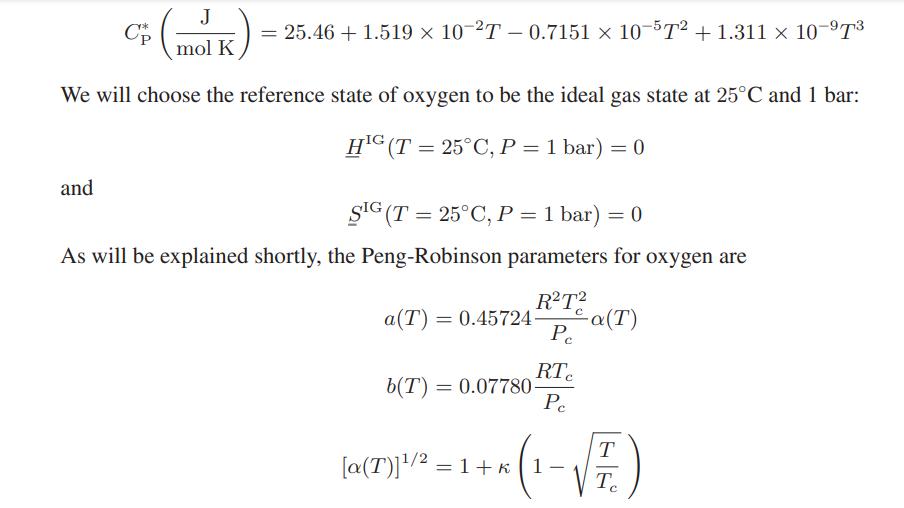
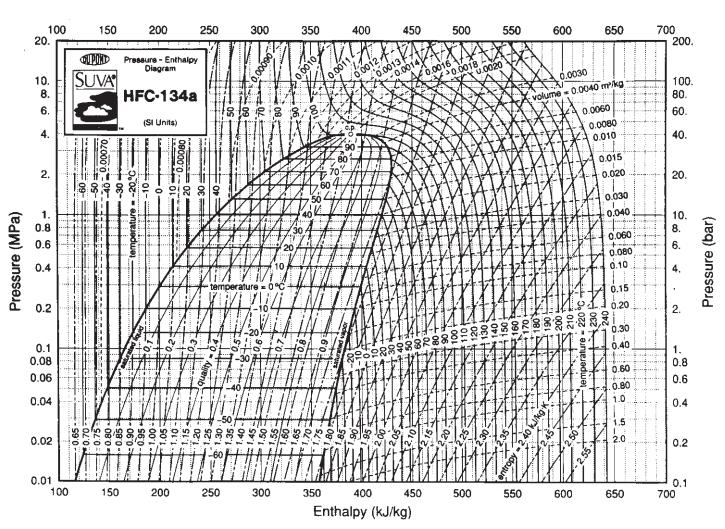
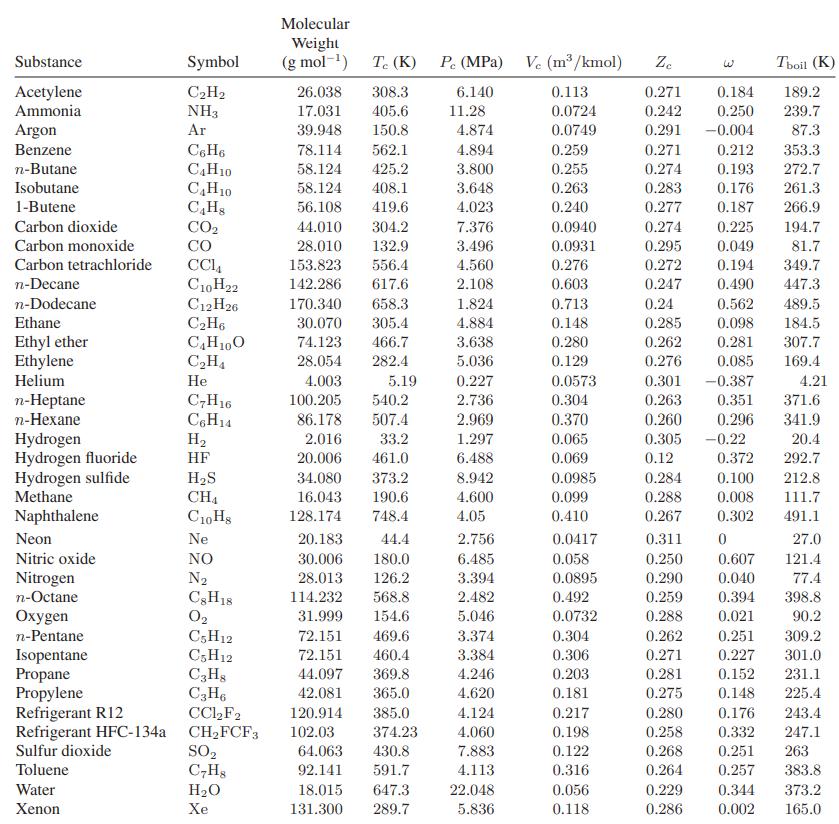
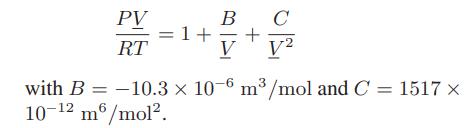
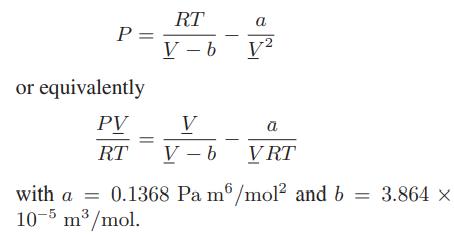
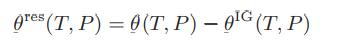
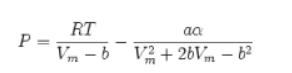
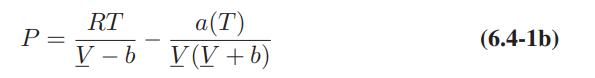
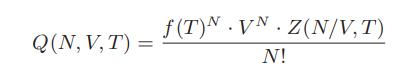
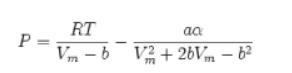
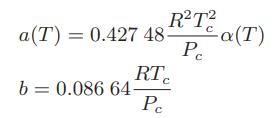
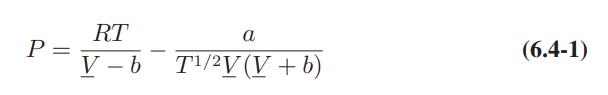
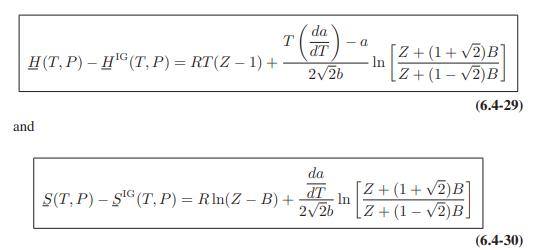
![T IG H(T, P) HG (T, P) = RT (Z 1) + and da dT 2/26 a In Z + (1+2)B] Z + (1 -2)B] da S(T, P) - SIG (T, P) =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/1/91365535c390d89a1699961909723.jpg)
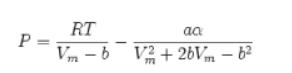
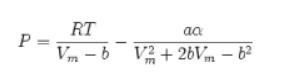
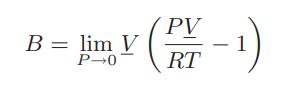
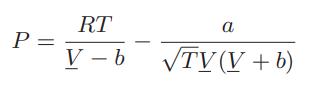
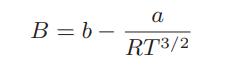
![= T . || - - -[1 - Ta]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/4/6836553670be66631699964680686.jpg)