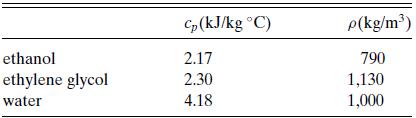
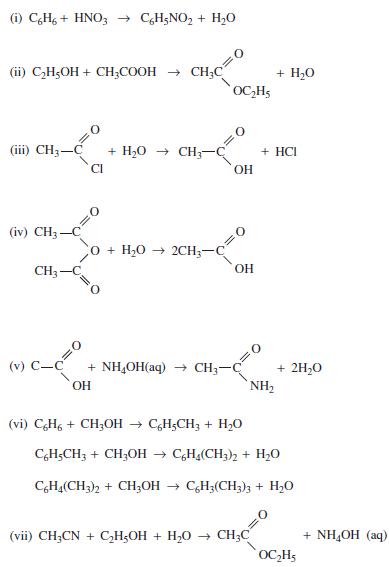
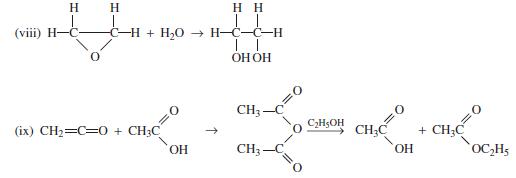
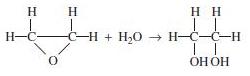![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![Qs h DL = = -Q hT DL = A e(1-A) L/B L 1 L [T,(x) T,;(x)]dx = AT0 + (Tio Tso) B (e(1-A)L/B - 1) - (1-A) L](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1697/5/3/0/871652e43f76425d1697530870115.jpg)
![ht DL (TL - TL) - ( ) - 450 = In [(TL - TL)/(To T)] - 10 = In(/) = .m:](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1697/5/3/0/896652e44109736b1697530895334.jpg)