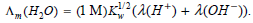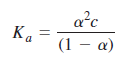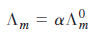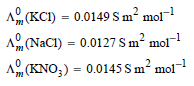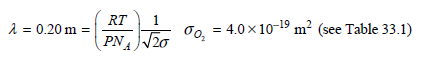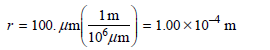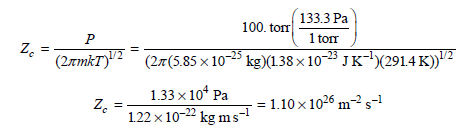![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


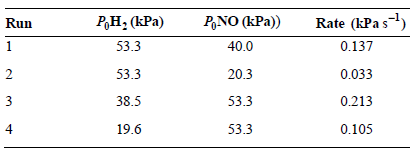
![Rate = H10,] [H*]2](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/5/3/8605afbd664c0d0f1526453855130.jpg)

![[н] (он ] ад аон 1м K. 1м](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/3/6/6/6755afa81d3011be1526366669622.jpg)