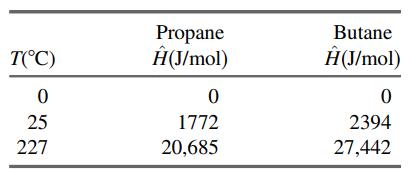
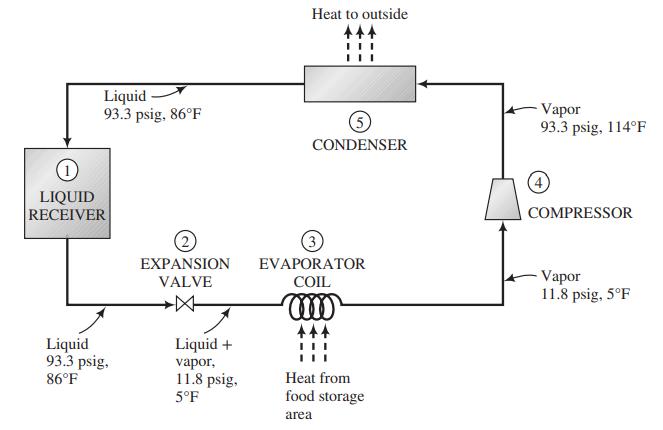
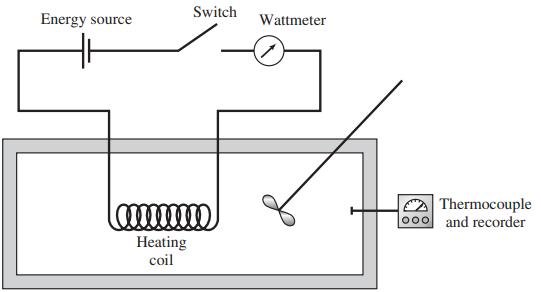
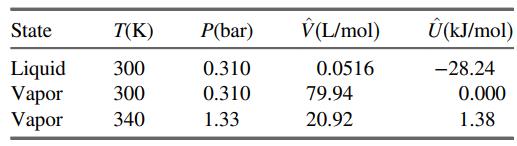
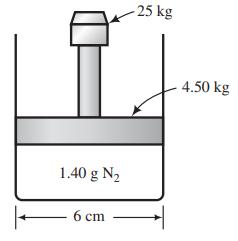
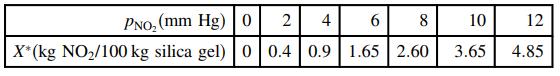
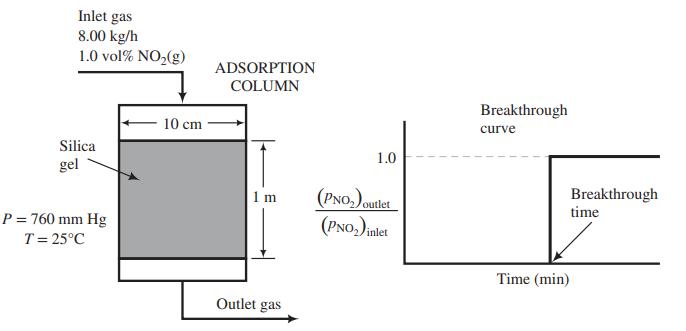
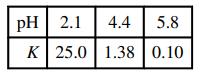
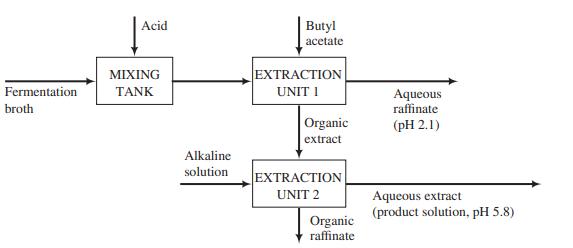
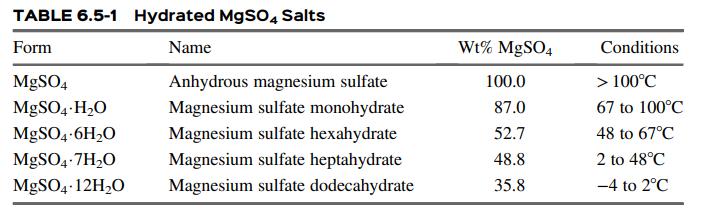
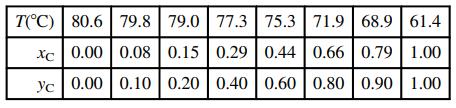
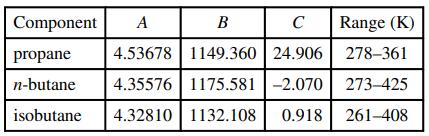
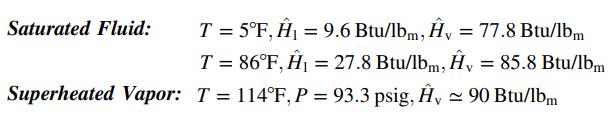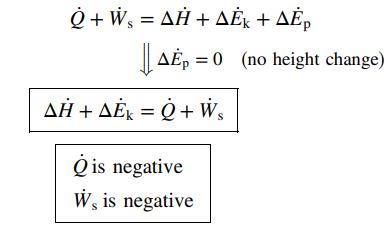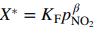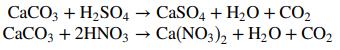![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


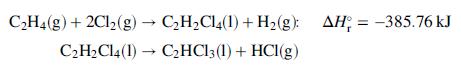
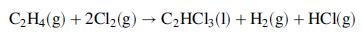
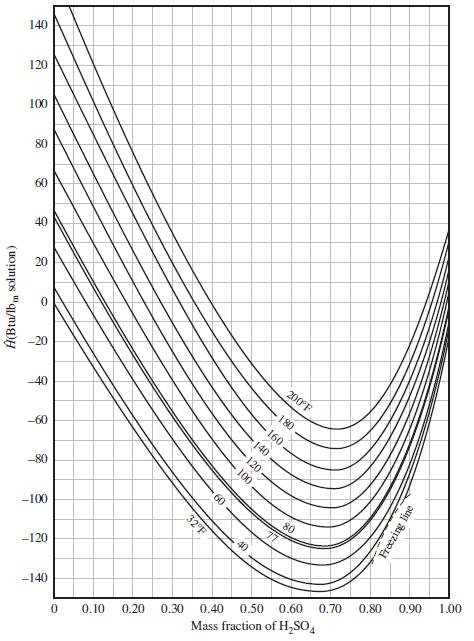
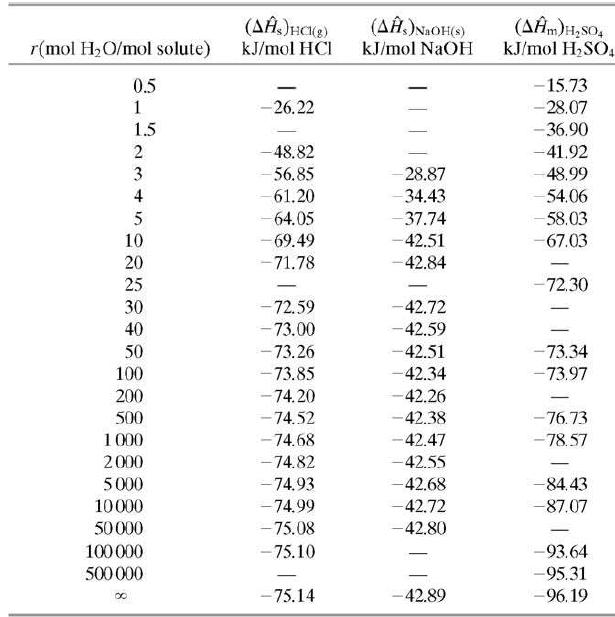

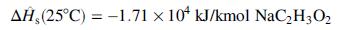
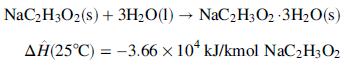
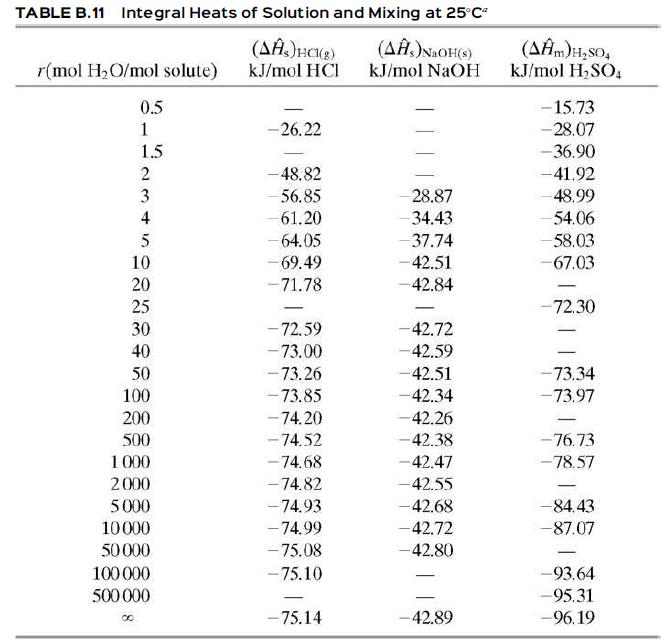
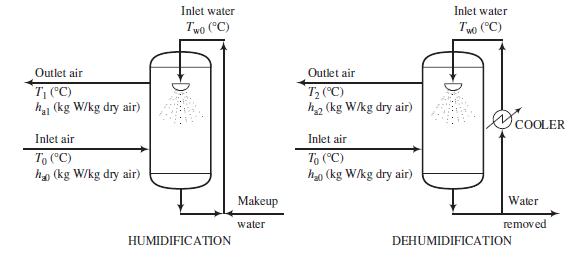
![Cp[H,O(s)] = 2.11 kJ/(kg- °C) Cp[H2O(v)]= 1.86 kJ/(kg. °C) Cp(oatmeal) = 1.5 kJ/(kg- °C) (AĤ %3D gubi tH,0 = 2845 kJ/kg](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/6/0/4/5515eceb3076fd3c1590604550781.jpg)
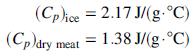
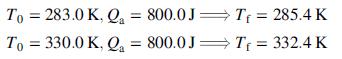

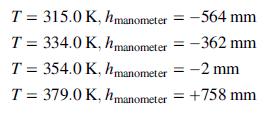
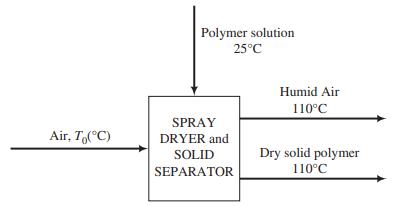
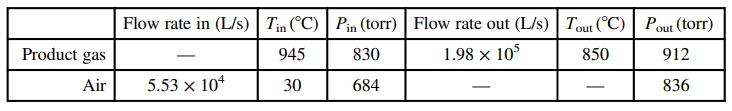
![Vapor product 65°C, P,(atm) References: P(I, 65°C), H(1, 65°C) Hin nin H out nout Yp[mol P(v)/mol] Substance P = pentane %3D H = hexane P(1) na ne FLASH 100 mol/s, liquid nd EVAPORATOR P(v) 50 mole% P ne ----- OkW) H(1) 50 mole% H 80°C, 5.0 atm H(v) Liquid product](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/8/2/8405ec6bd18137661590082835384.jpg)
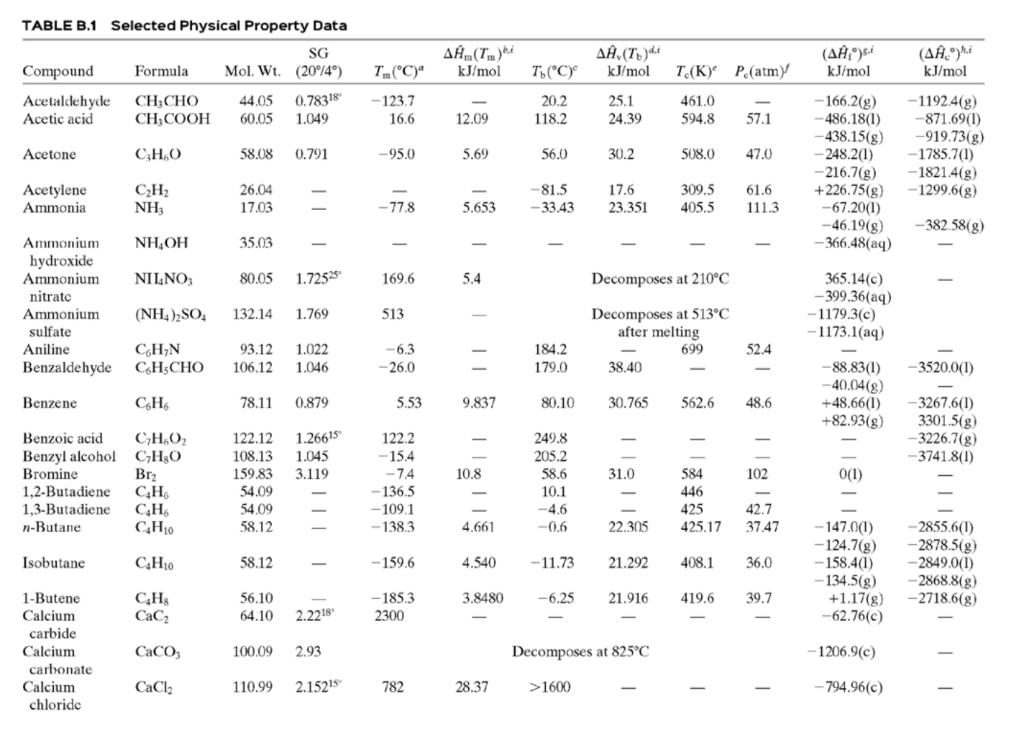
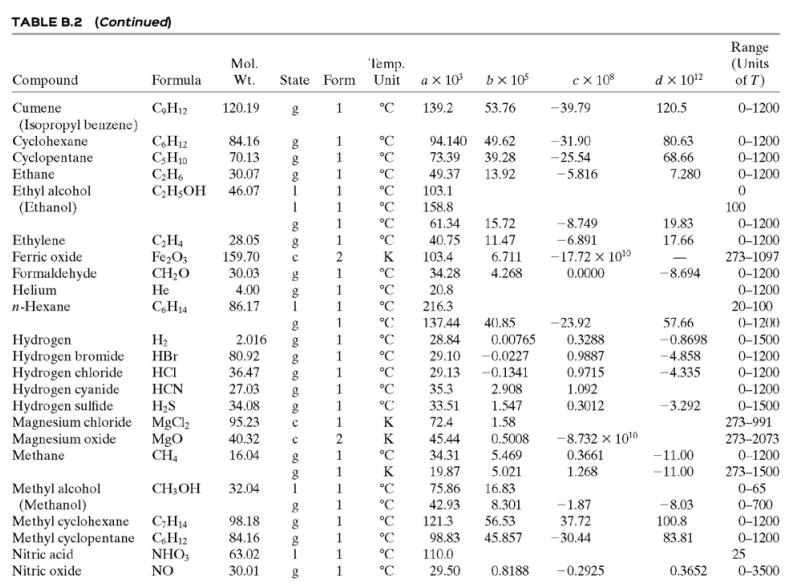
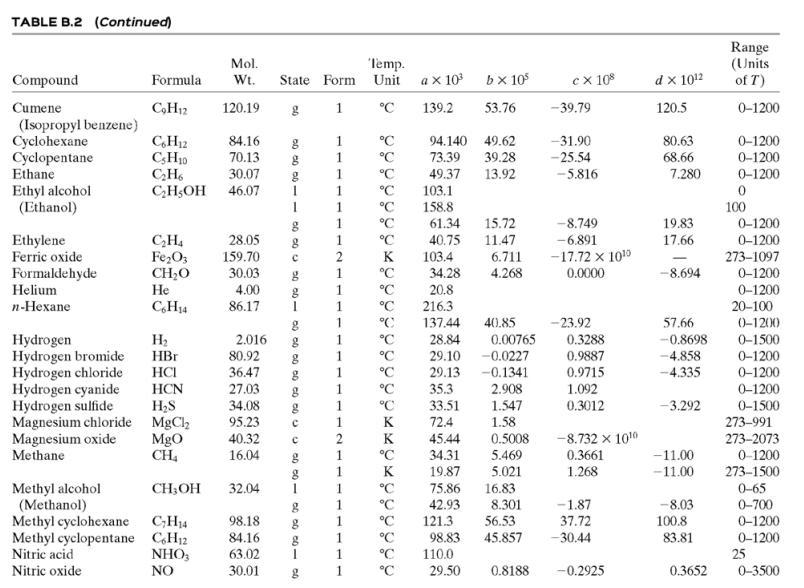
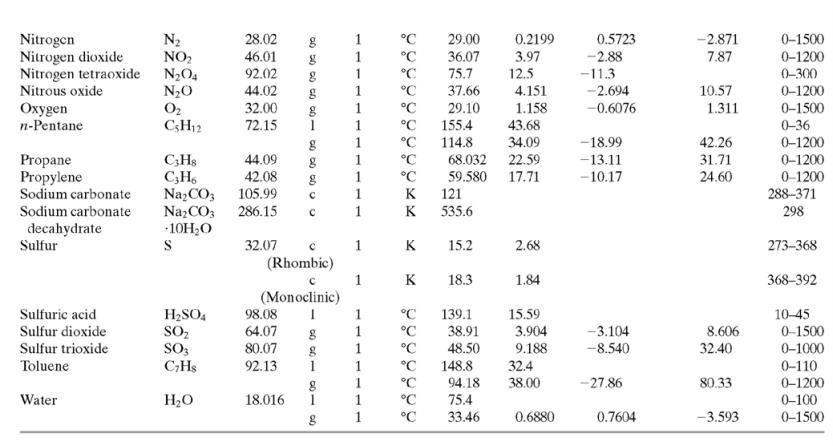
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/8/2/6645ec6bc68ba9271590082649906.jpg)
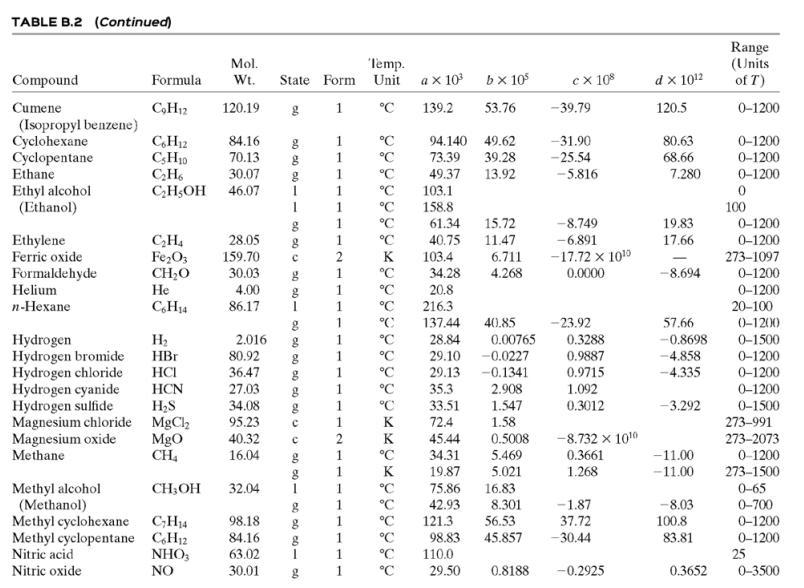
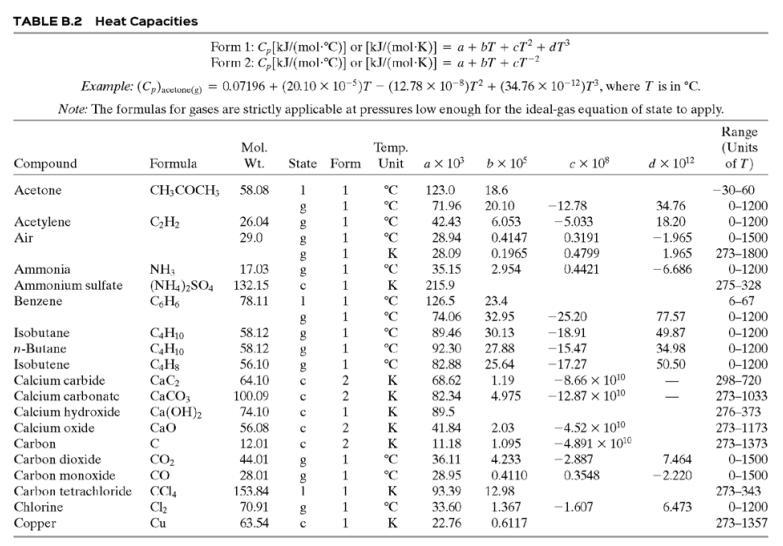
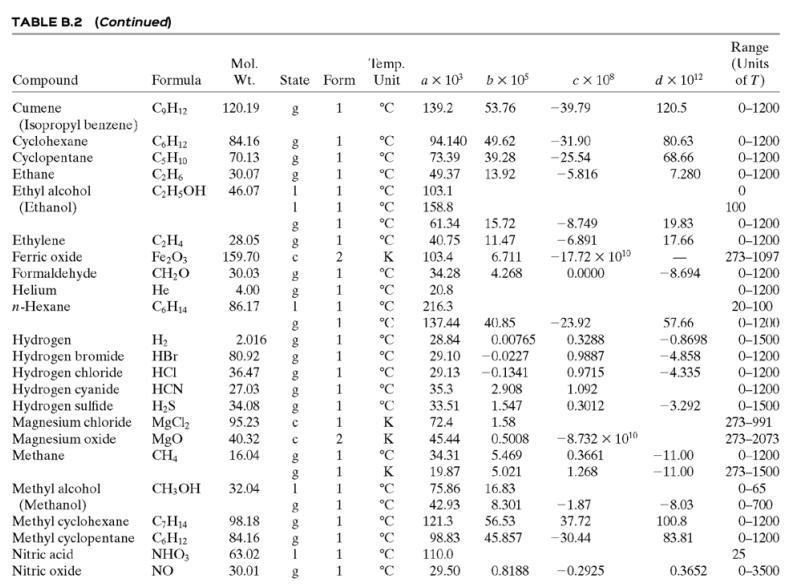
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/6/3/4035ec6712b530b51590063382161.jpg)
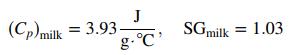
![0.7 0.6 0.4 0.2 350 525 600 800 1000 Wavelength [nm] Abs](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/5/9/5345ec6620e7b4f21590059528903.jpg)
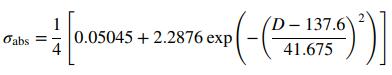
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol·K)] = a + bT + cT + dT Form 2: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cT? Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-$)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/5/6/4075ec655d7a186e1590056399297.jpg)