![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


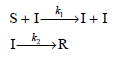
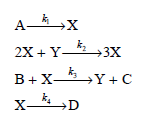
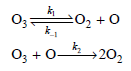
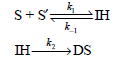
![d[HBr]_ k[H2][Br,]2 тHBr] dt 1+ [Br,]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/3/0085afbfa20b923f1526463004273.jpg)
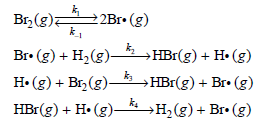
![d[HBr] 1/2 [H,],[Br, }? ks = 2k2 t=0 dt](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/3/1535afbfab1a56331526463150069.jpg)
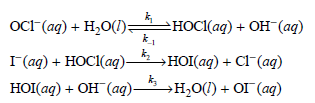
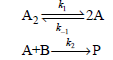
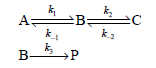
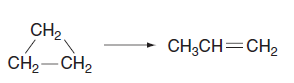
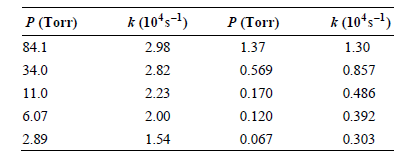
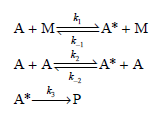
![k;(k[A][M] + k,[A]) K[AJ) k1[M] + k_q[A] + k_3 R =](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/3/6965afbfcd0016621526463658616.jpg)
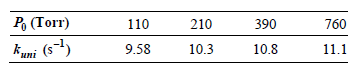
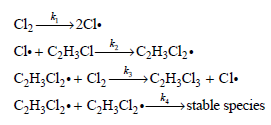
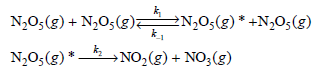
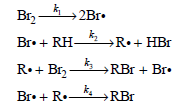
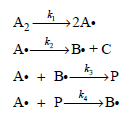
![[H,0,], (M) 0.001 0.002 0.005 Initial Rate (M s) 1.38 x 10-3 2.67 x 10-3 6.00 x 10-3](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/4/1375afbfe89ca8c71526464136149.jpg)
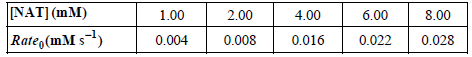
![NBT] (mM) 1.00 2.00 4.00 6.00 8.00 Rateg(mM s) 0.062 0.107 0.040 0.082 0.099](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/4/3245afbff4459dcf1526464313988.jpg)
![[S] (AM) Rateo (pM s), ц - 0 Rateo (uM s), 1 % 200 иМ %3D 0.299 0.071 0.018 0.500 0.100 0.030 0.820 0.143 0.042 1.22](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/4/4865afbffe6c25b31526464482646.jpg)
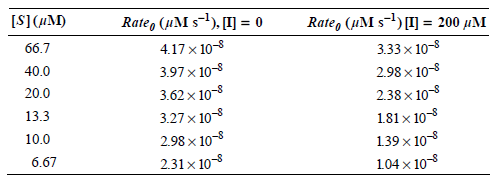
![Rmer[S]. Ro [S], + Km max <aעל .](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/5/1925afc02a8d8bd11526465189326.jpg)
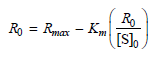
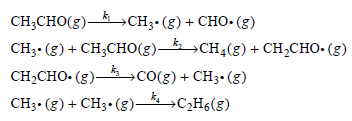
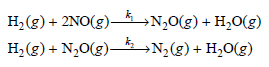

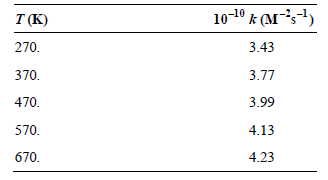
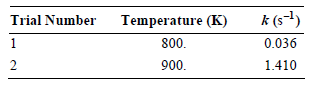
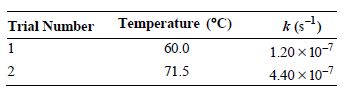
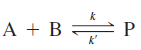
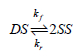
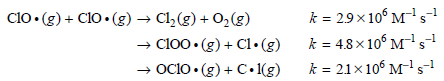
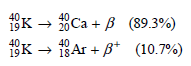
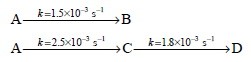
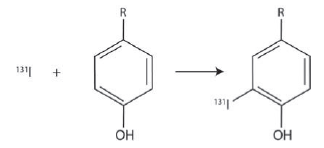
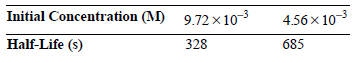
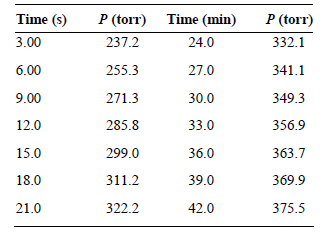
![[lactose], (M³) 0.01 [H*](M!) 0.001 Initial Rate (Ms) 0.00116 0.00232 0.02 0.001 0.01 0.00464 0.004](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/5/4/4425afbd8aa9207b1526454439615.jpg)
 1.0 x 101 1.0 ×101 3.0x 101 [03](cm-³) 1.0 x 1012 5.0 x 1012 1.0x 1012 19x 108 9.5 x](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/5/4/3715afbd8634b5f31526454364697.jpg)