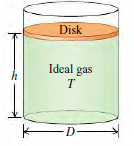
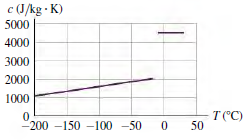
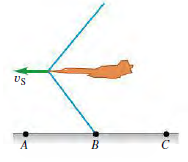
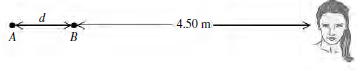![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


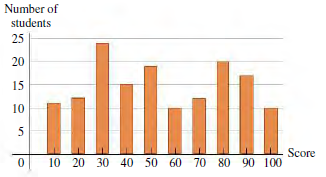

![Coefficients of Volume Expansion Solids В (к- оr (С)-1] Liquids B [K-! or (C°)-'] 75 x 10-5 115 x 10-5 Aluminum 7.2](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1539/3/5/5/5275bc0b38780a3c1539381175303.jpg)