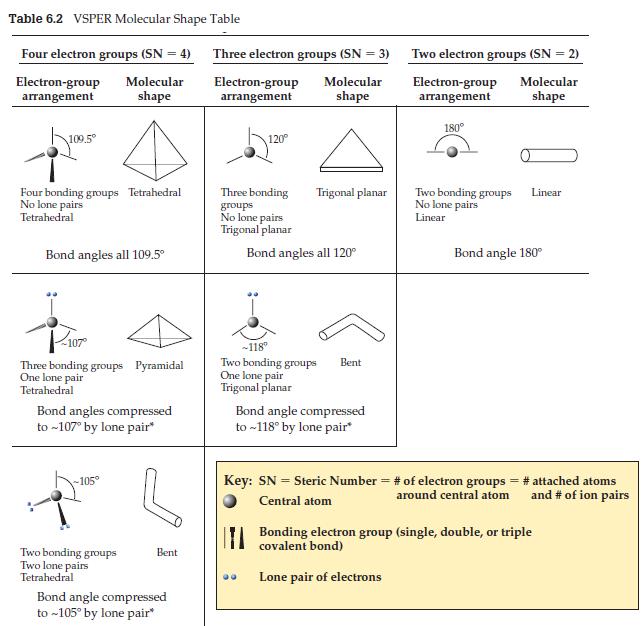
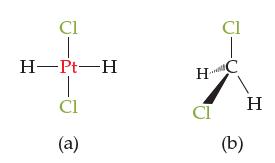
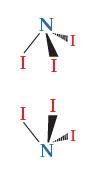
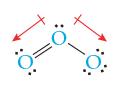
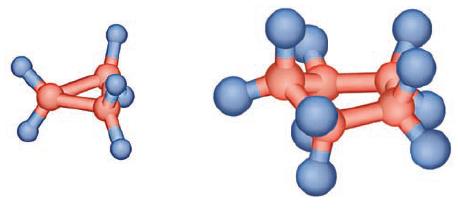
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


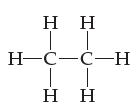
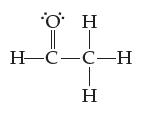
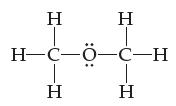
![[H-Q-H]+ -C H](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/4/1/9/0386569981e489161701419037757.jpg)
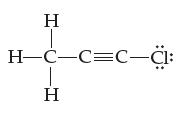
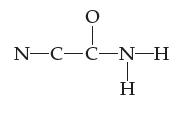
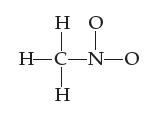
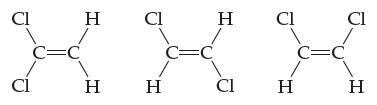
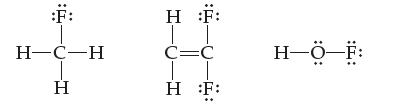
![H-Z: H-N: [H_N_H]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/4/2/0/77265699ee4a53f81701420770333.jpg)