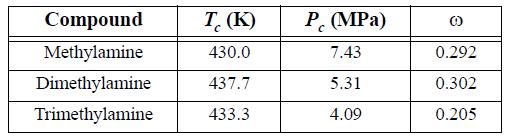
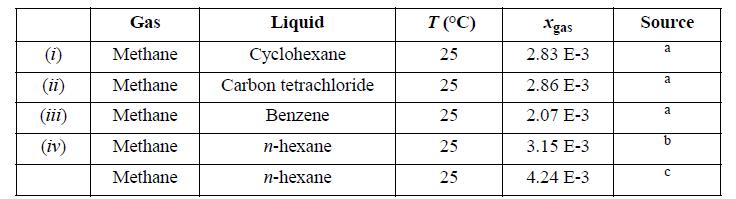
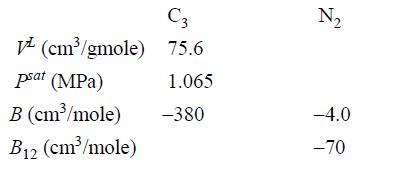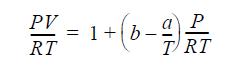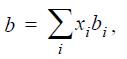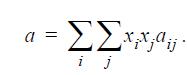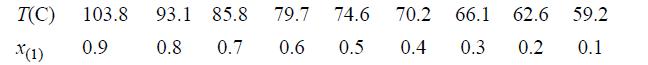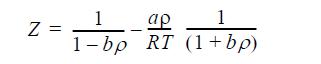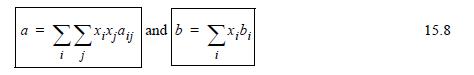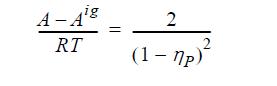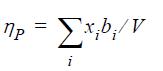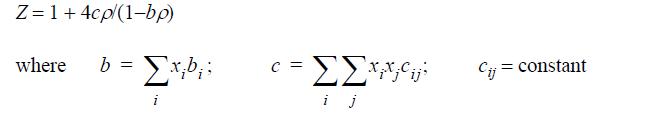![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


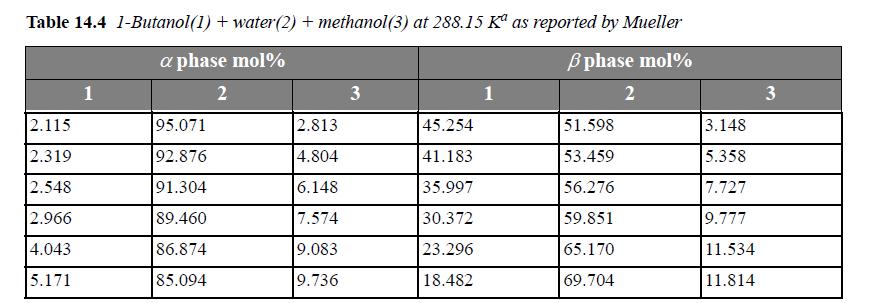
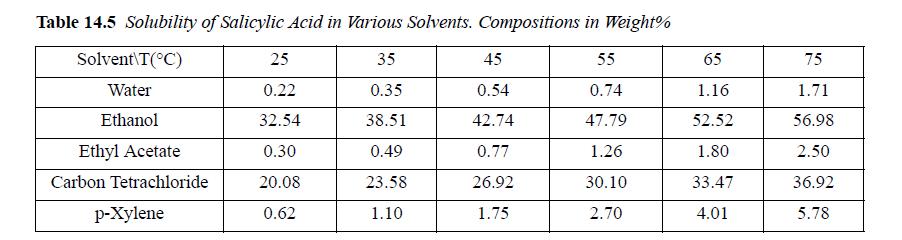
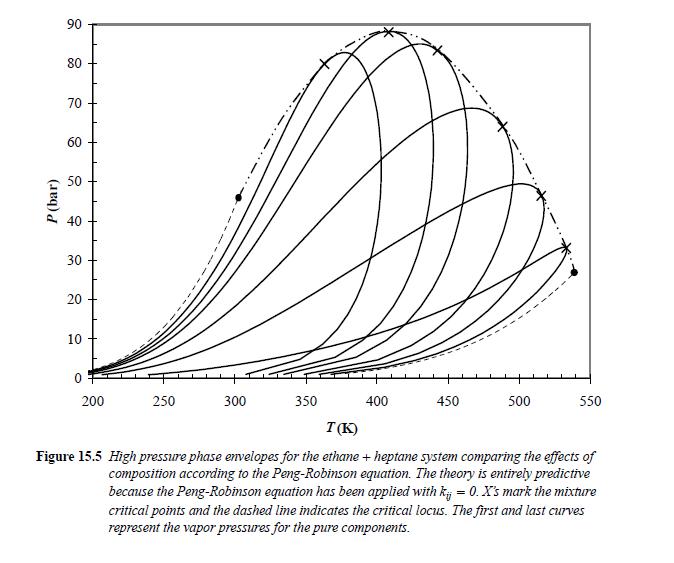
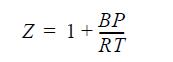
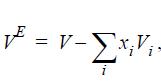
![Z= 1 + 4cbp/(1-bp) where b = C = Exibi i Cij = cii C jj bk (ANS. In ok = Ok = 4(c-2[x;,,x]hu(1- bp) + (2-1)-](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1696/2/3/2/636651a74bc2c18b1696232635996.jpg)