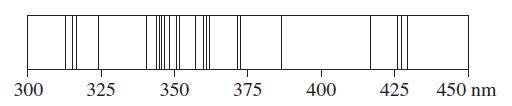
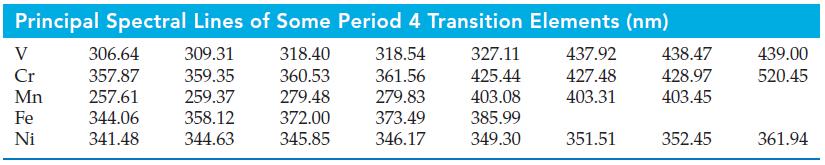
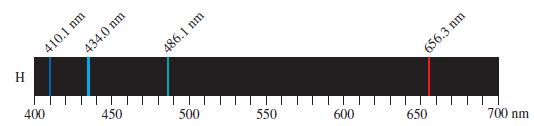
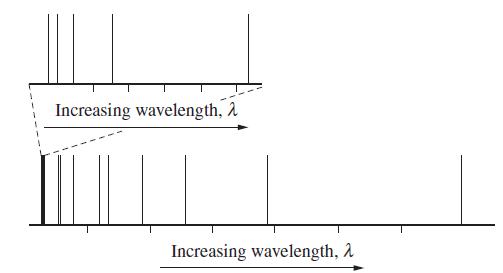
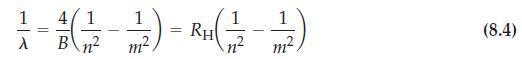![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


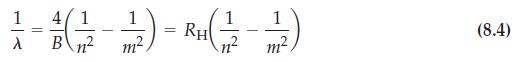
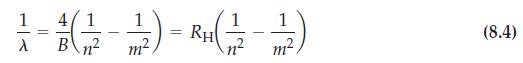
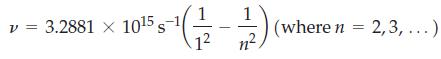

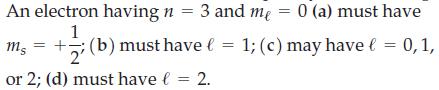
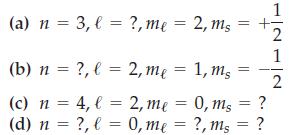
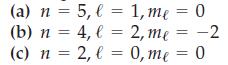
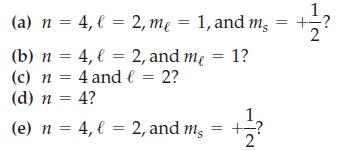
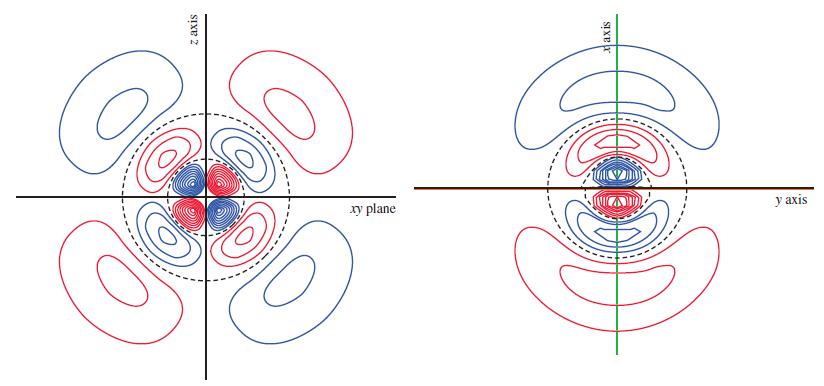
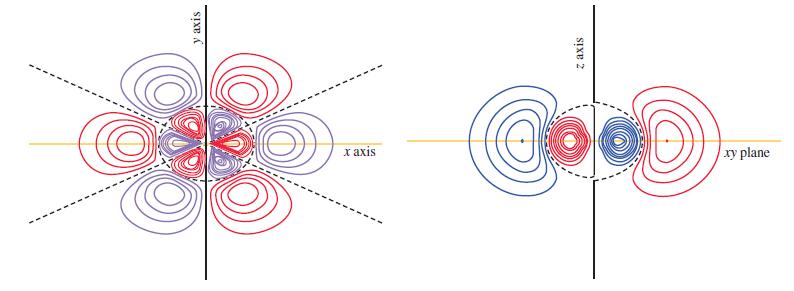
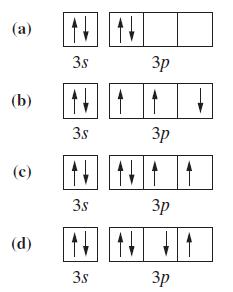
![(a) [Ne] 3s (b) [Ne] 14 3s (c) [Ne] (d) [Ne] 3s 3.s 11 3 3 3 3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/9/3/659654f445bcb61f1699693660384.jpg)
![(a) [Ar] (b) [Kr] (c) [Kr] (d) [Ar] NNNNN 3d 4d 4d 3d 5s 5s 3f 4s N1 4p 4d](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/9/3/683654f44739734d1699693684113.jpg)
![(a) [B] (b) [C] (c) [N] (d) [O] 1s 25 N N 1s 25 2p 2p 1s 25 2p N N ^^ 1s 2s 2p](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/9/4/024654f45c8d2efd1699694025066.jpg)