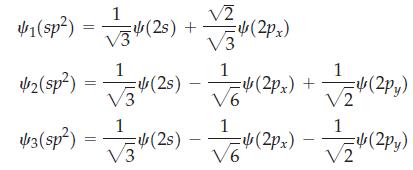![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


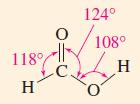
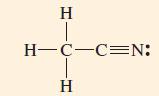
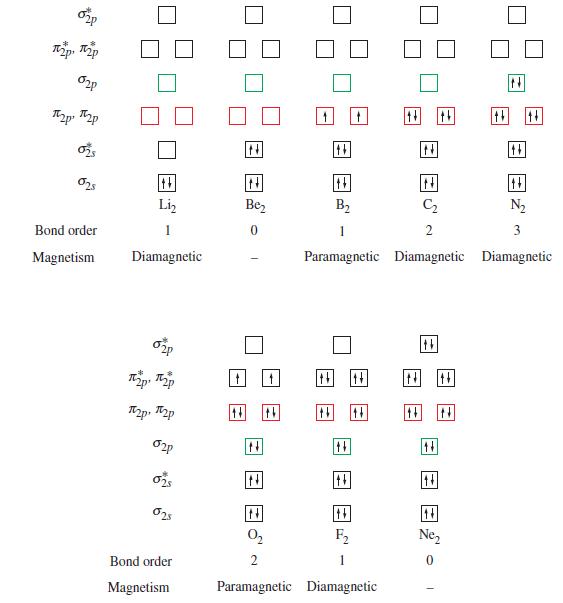
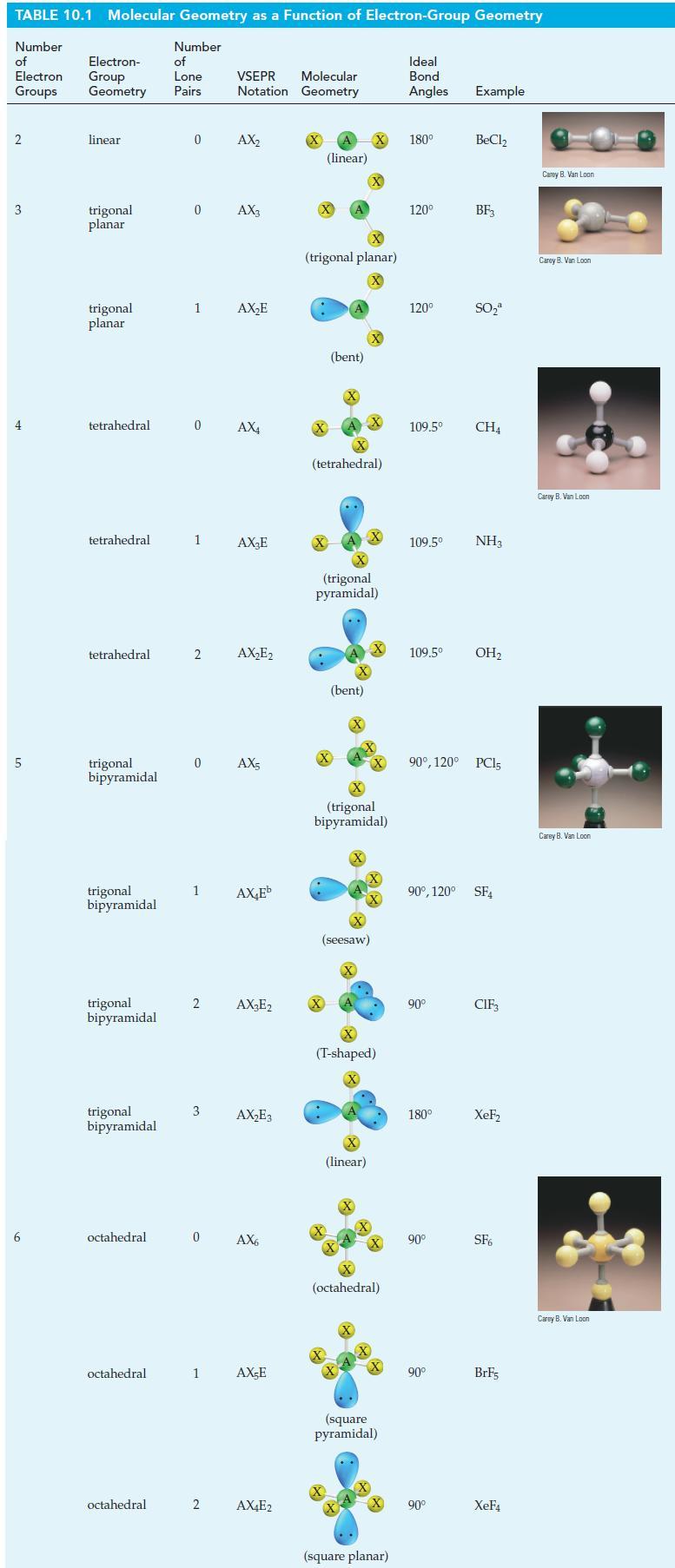
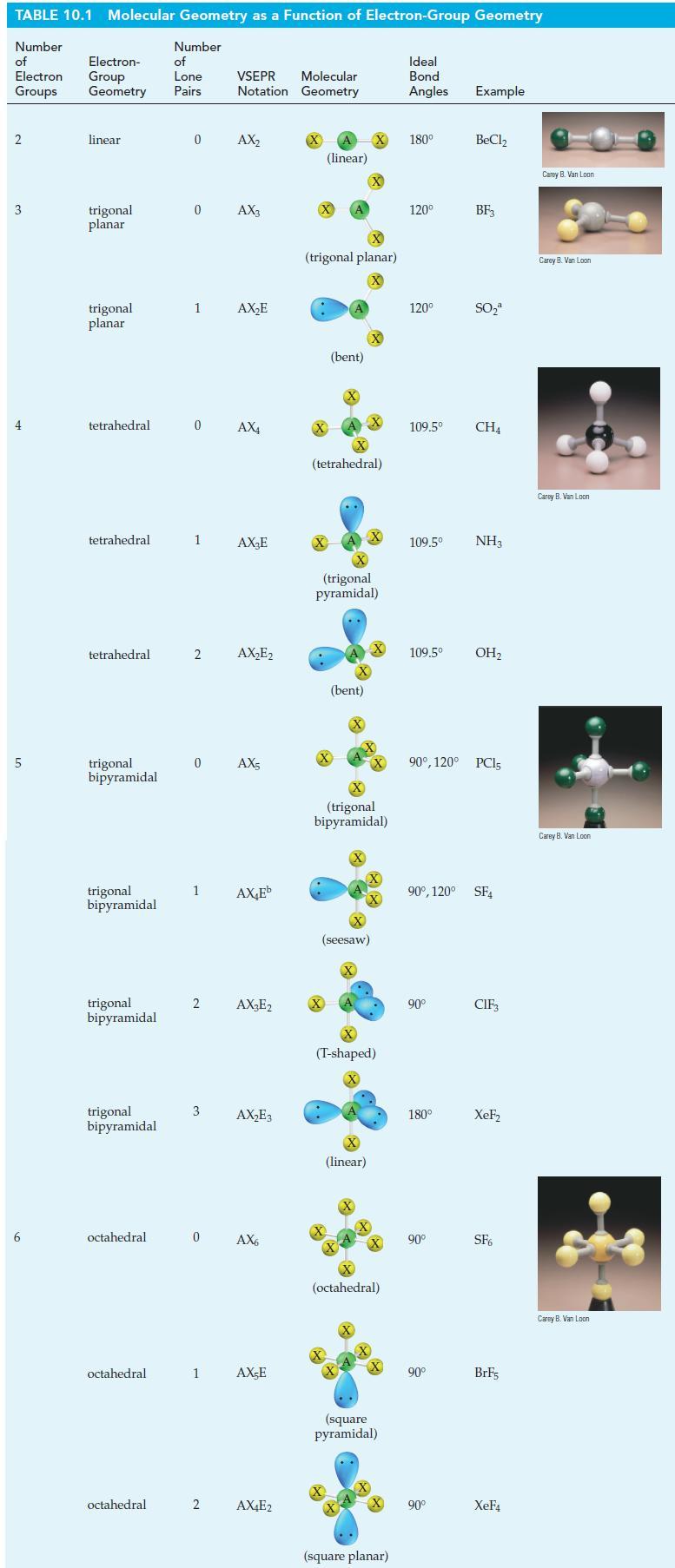
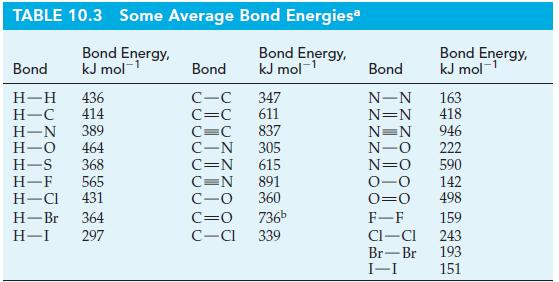
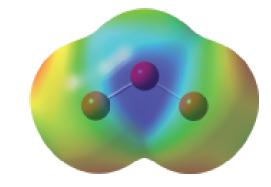
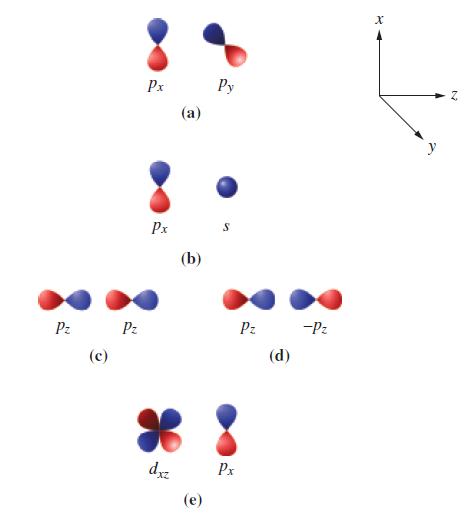
![T: (2p)-0(2p) H o: H(1s)-C(sp) 120C= H 120 o: C(sp)-0(2p) [or, o: C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/5/7/32465534a4c3eead1699957323151.jpg)
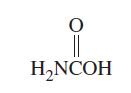
![: T: C(2p)-0(2p) H H(1s)-C(sp) 120 C- H 120 o: C(sp)-0(2p) [or, : C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/2/58765535edb4ceb11699962586090.jpg)
![T: C(2p)-0(2p) H o: H(1s)-C(sp) 120C- H 120 : C(sp)-0(2p) [or, o: C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/2/79665535fac55c581699962795141.jpg)
![T: C(2p)-0(2p) H o: H(1s)-C(sp) 120 C H 120 o: C(sp)-0(2p) [or, o: C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/2/9316553603328d4b1699962929959.jpg)
![T: C(2p)-0(2p) H o: H(1s)-C(sp) 120 C H 120 o: C(sp)-0(2p) [or, o: C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/2/9226553602a09d1f1699962920805.jpg)
![T: C(2p)-0(2p) H o: H(1s)-C(sp) 120 C H 120 : C(sp)-0(2p) [or, o: C(sp2)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/3/26865536184657021699963267134.jpg)
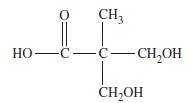
![TT: C(2p)-0(2p) H r: H(ls)C(sp2) _120 C-8 H 120 0: C(sp)-0(2p) [or, o: C(sp2)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/3/390655361fe1aa241699963388814.jpg)
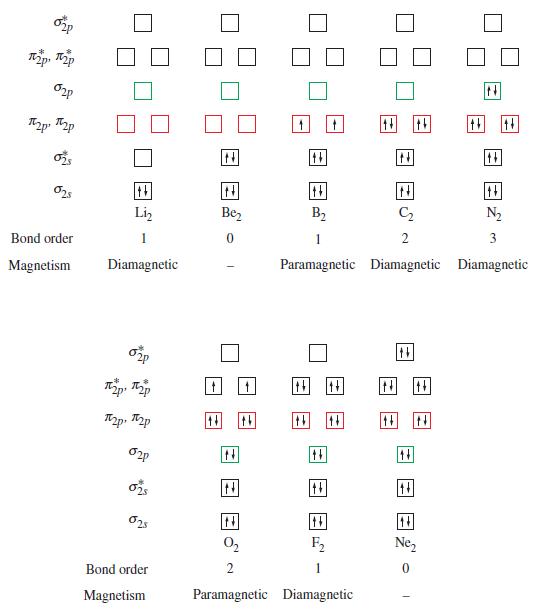
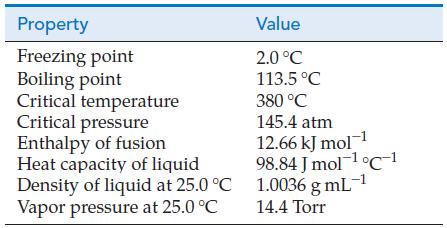
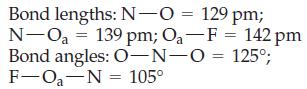
![T: C(2p)-0(2p) H o: H(1s)-C(sp) 120 C- H 120 : C(sp)-0(2p) [or, o: C(sp2)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/6/21965536d0b3a9571699966217921.jpg)
![: T: C(2p)-0(2p) H H(1s)-C(sp) 120 H 120 0: C(sp)-0(2p) [or, o: C(sp)-0(sp)]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/6/85965536f8b1ff951699966857852.jpg)
![$1(sp): 14 (25) [4(2s) +(2pz)] 1 42(sp) = [4 (25) 4 (2p)] -](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/0/2/0/03365543f41995851700020031923.jpg)