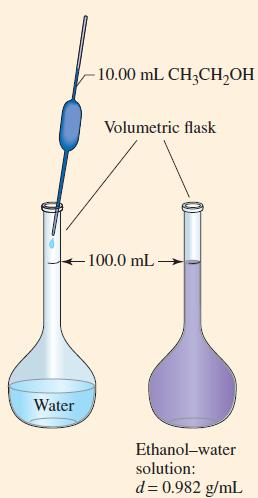
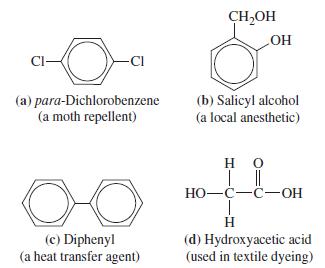
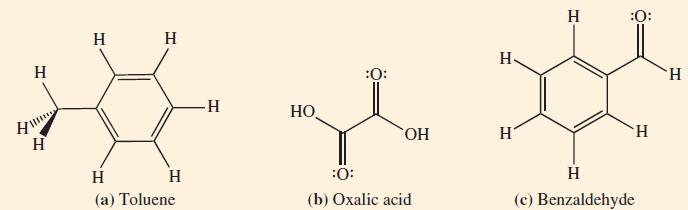
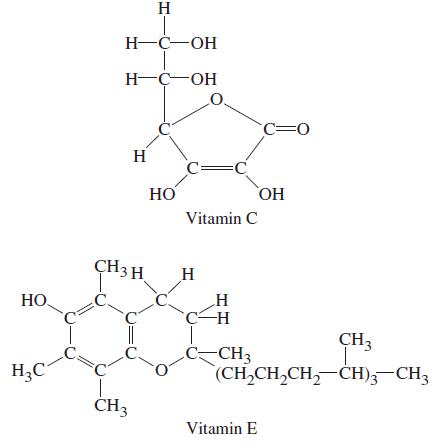
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


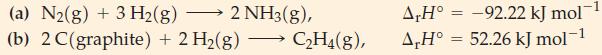
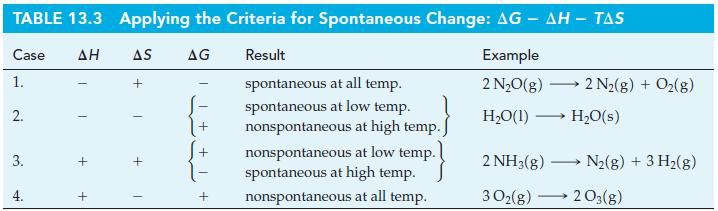


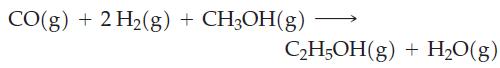
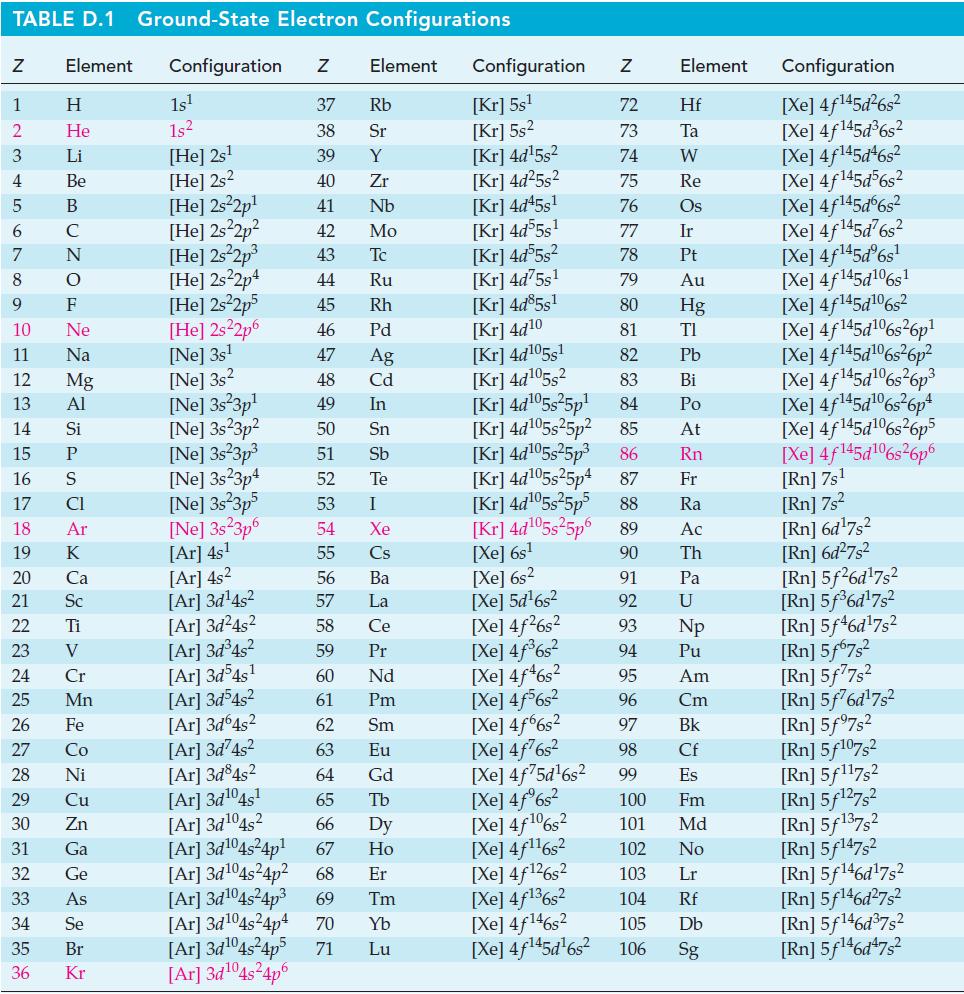
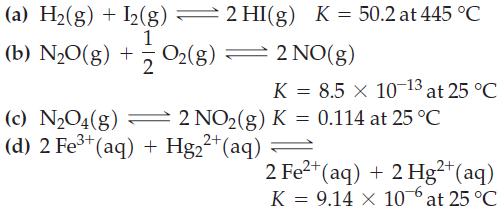
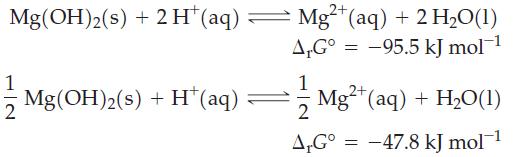
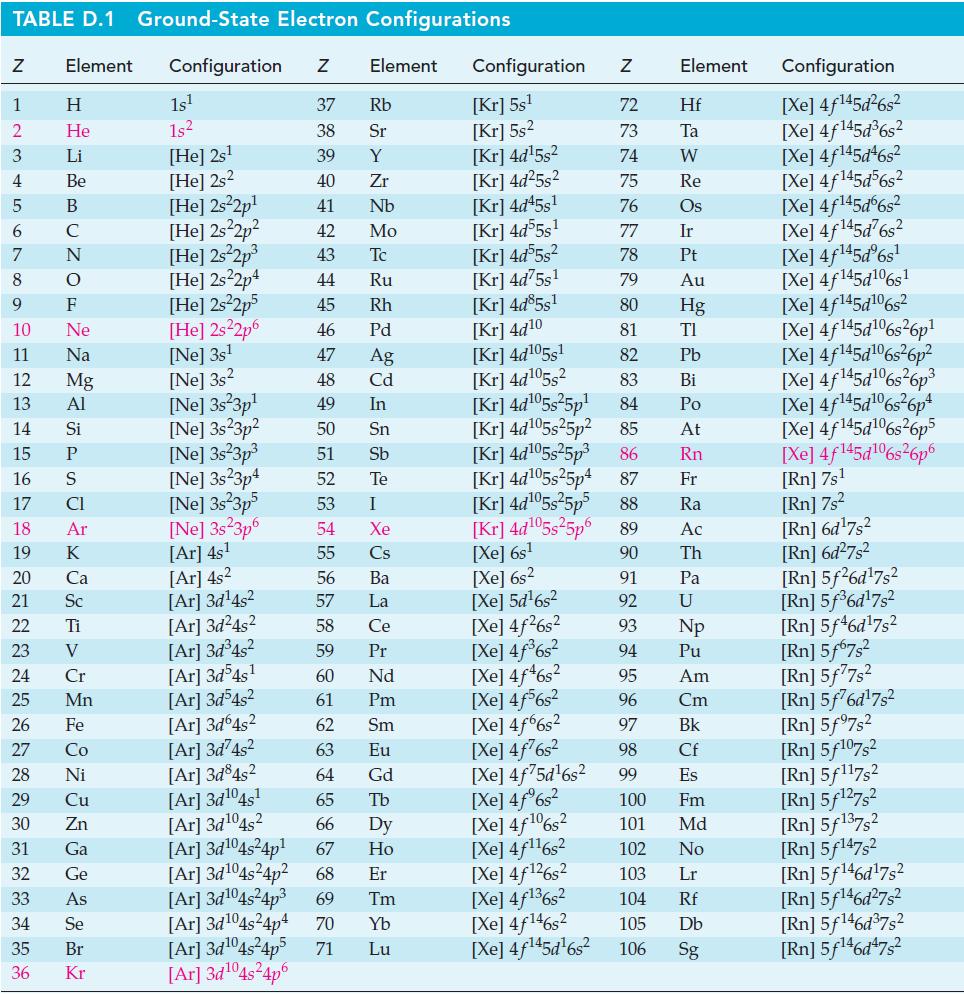
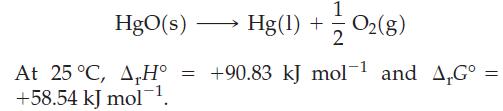
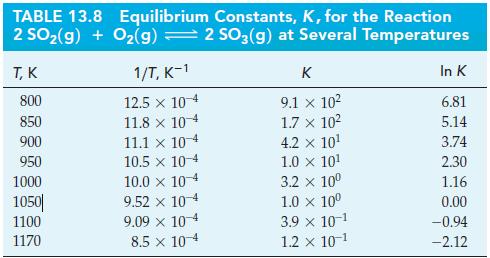
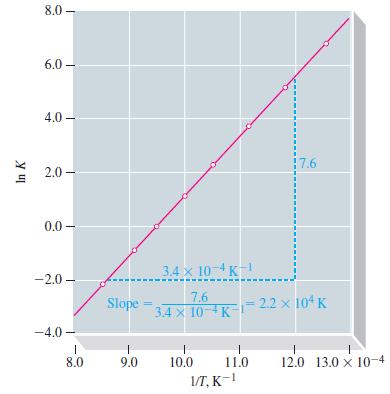
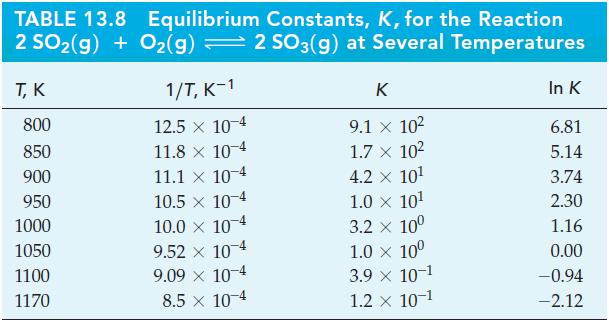
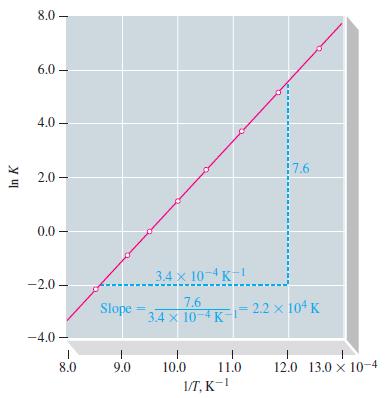
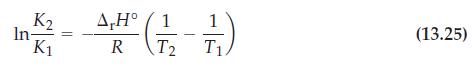
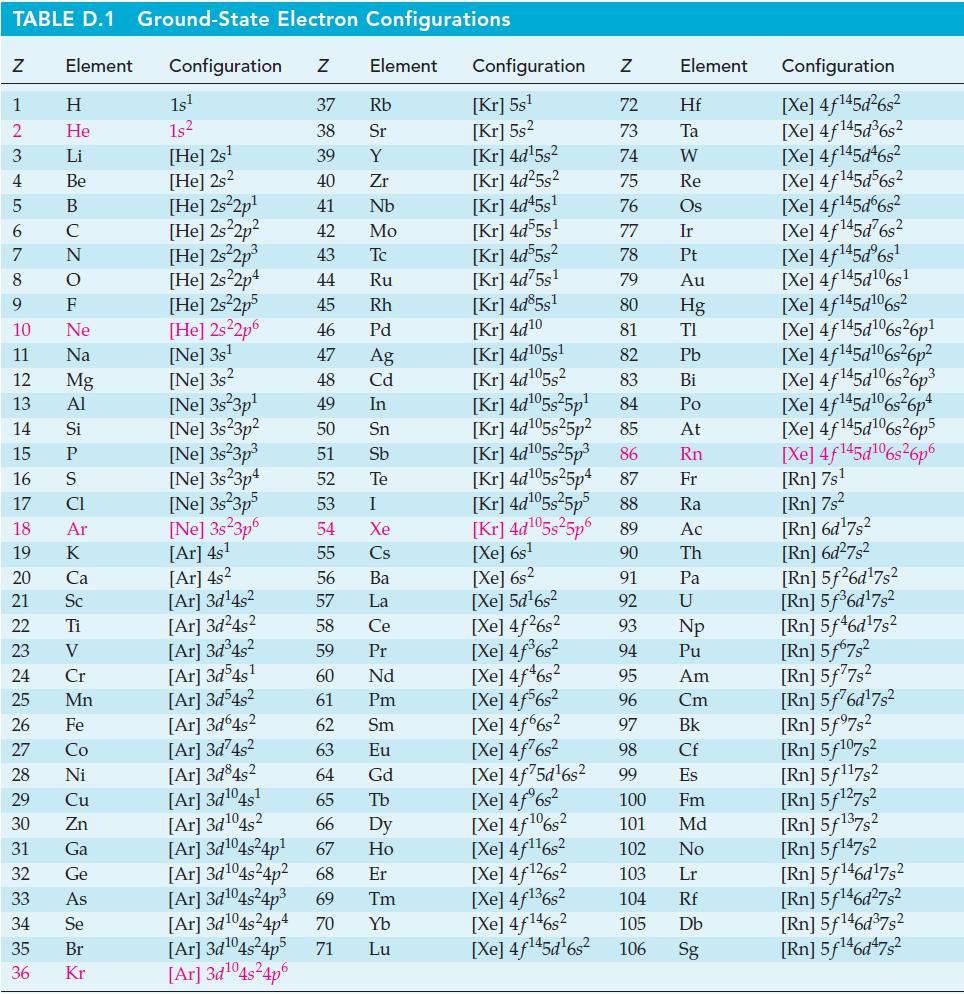
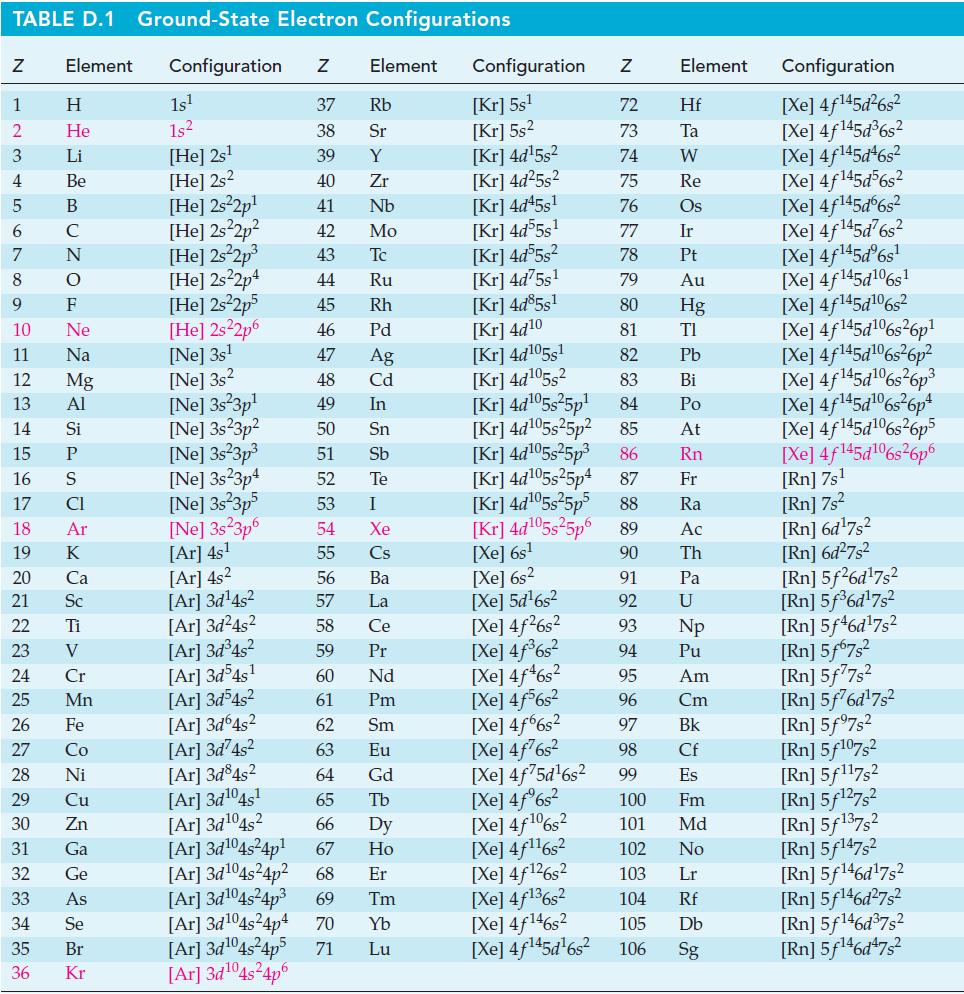
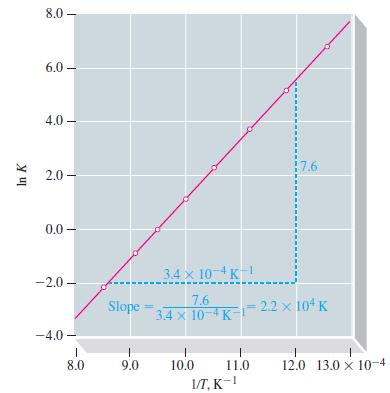
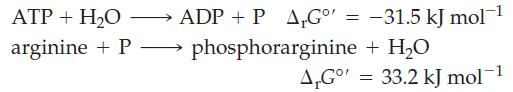




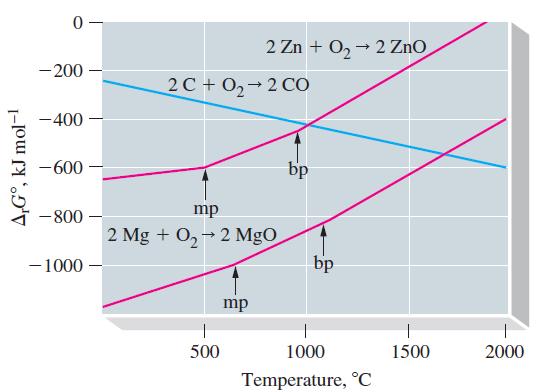
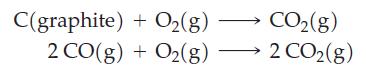
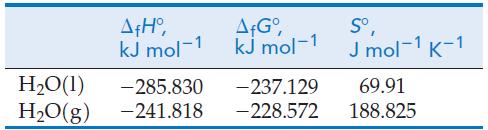
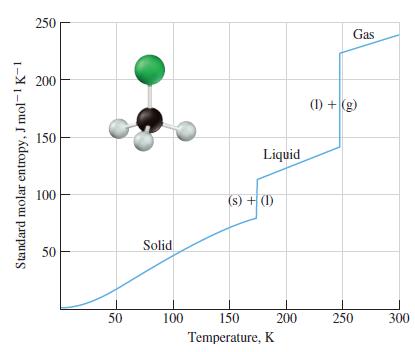
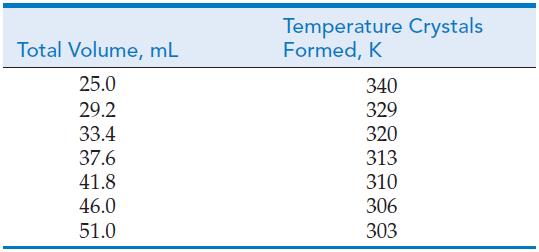
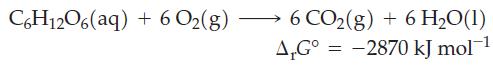
![Pco = 0.050 bar; Po = 0.132 bar; [glucose] = 1.0 mg/mL; pH = 7.0; [ATP] = [ADP] = [P] = 0.00010 M.](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/2/3/180655758cc99c891700223180209.jpg)