![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


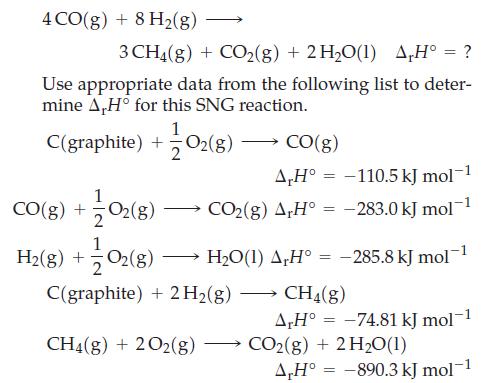
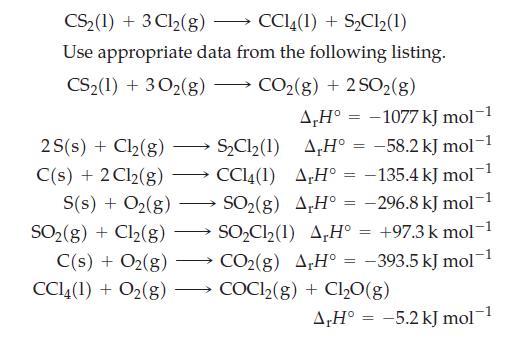
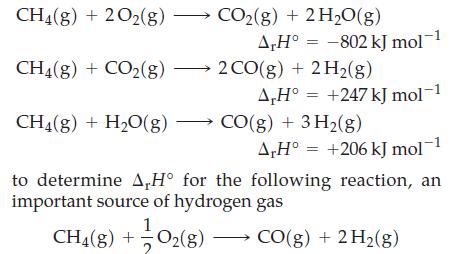
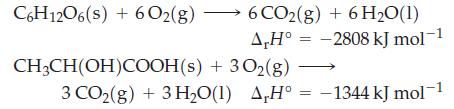
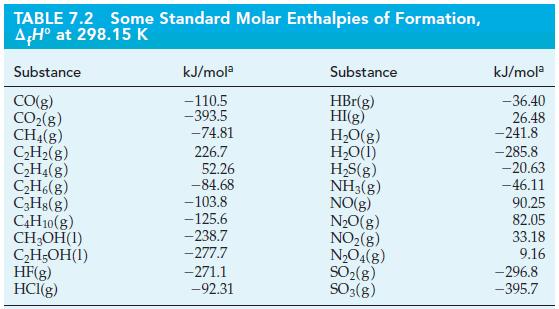
![A,H [cx AHc + dx AHD + ...]-[ax AHA + bx AHB +...] (7.22) weighted sum of A H values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/241654de711110c61699604236540.jpg)
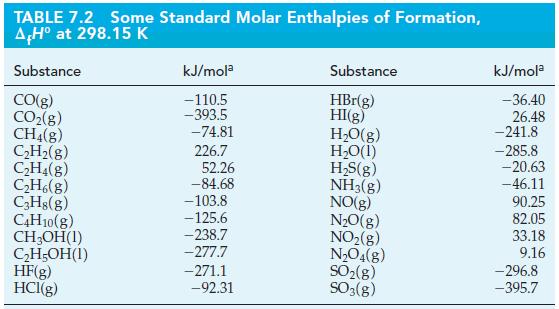
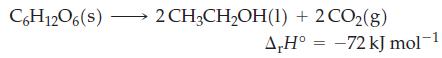
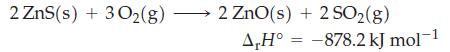
![A.H = [cx AHC + dx AHD +...] [ax AHA + bx AHB + .] (7.22) weighted sum of AcH values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/689654de8d1674691699604684867.jpg)
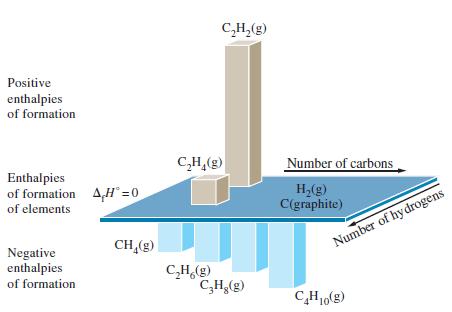
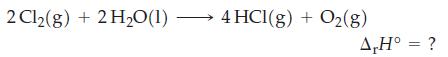
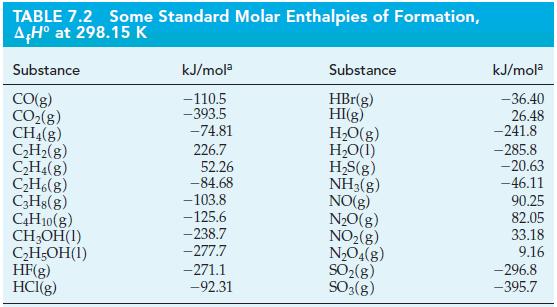
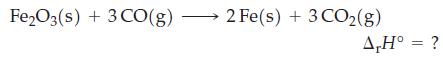
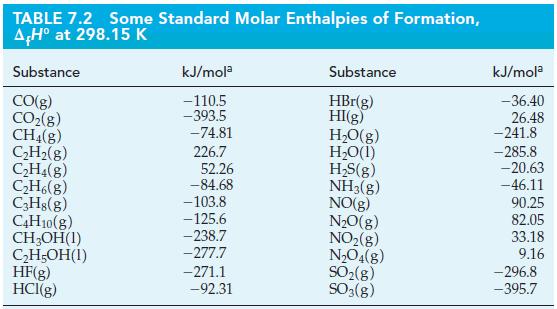
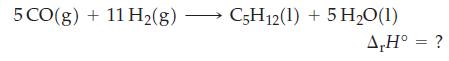
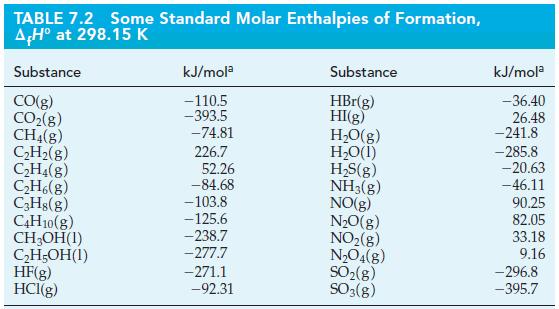
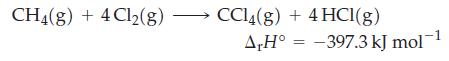
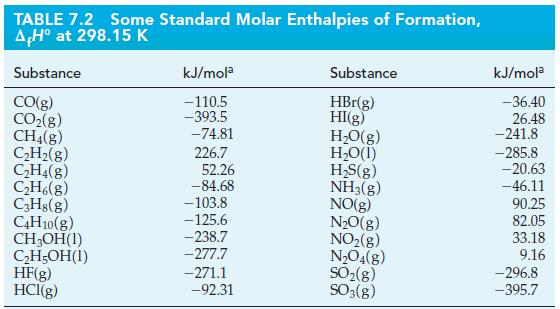
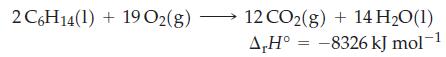
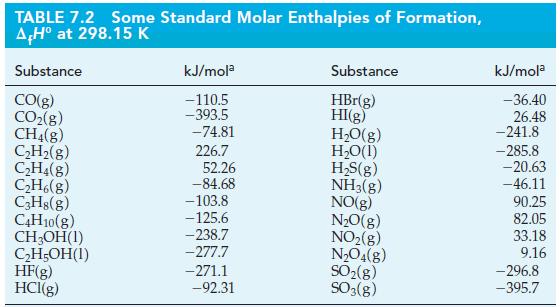
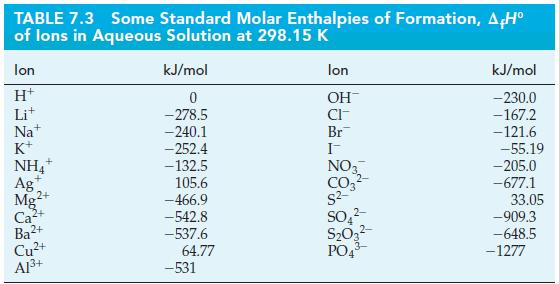
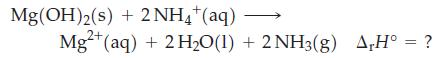
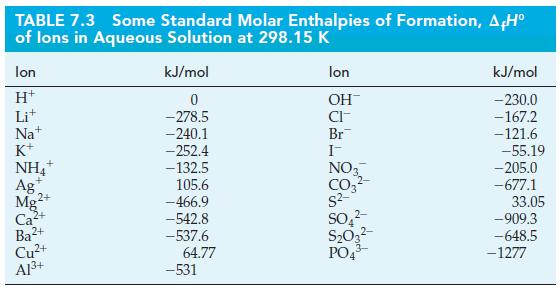
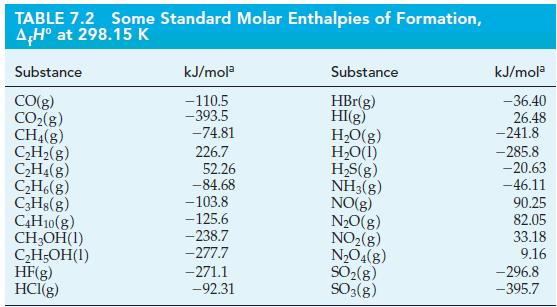
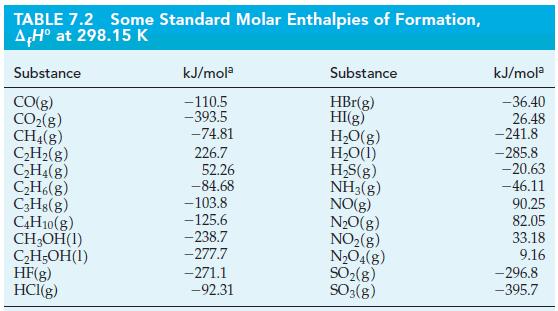
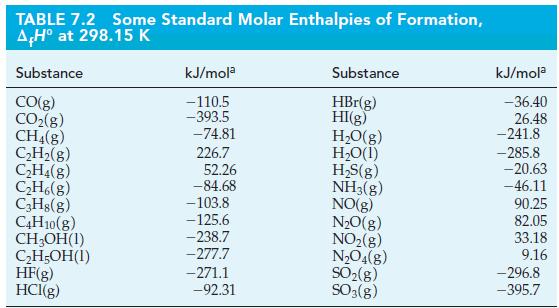
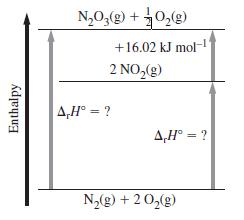
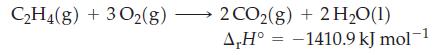
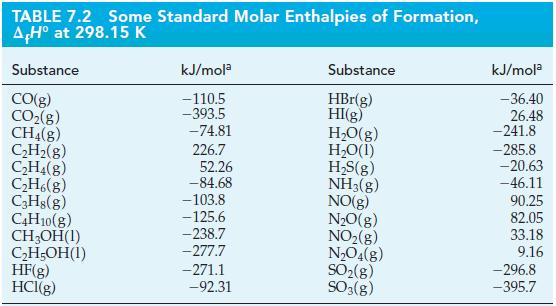

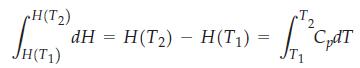
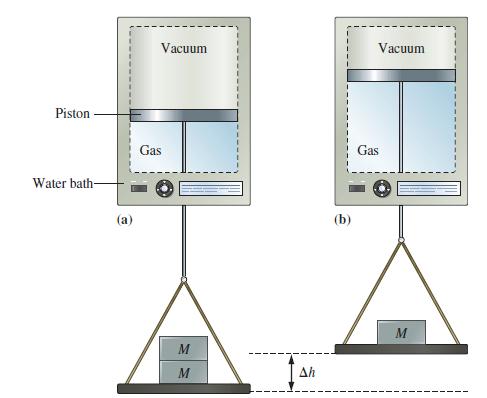
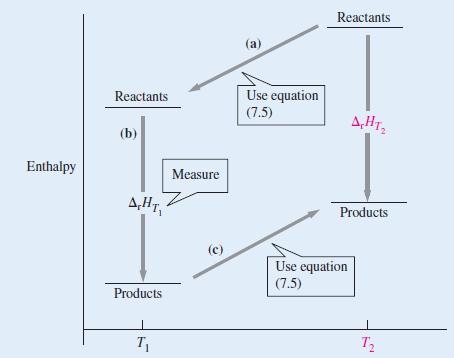
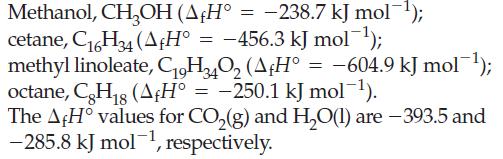
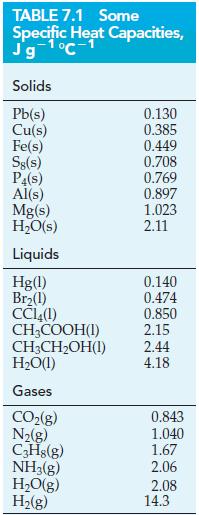
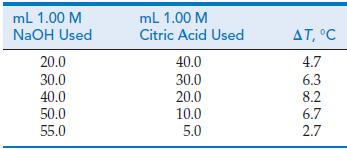
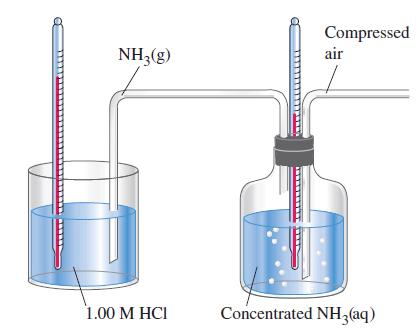
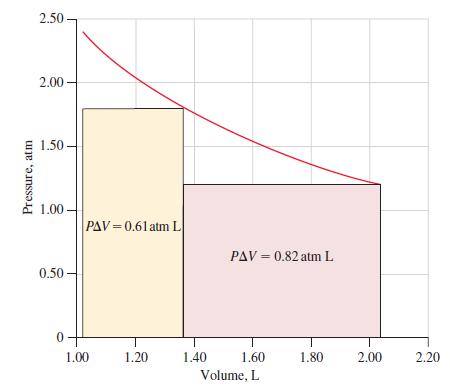
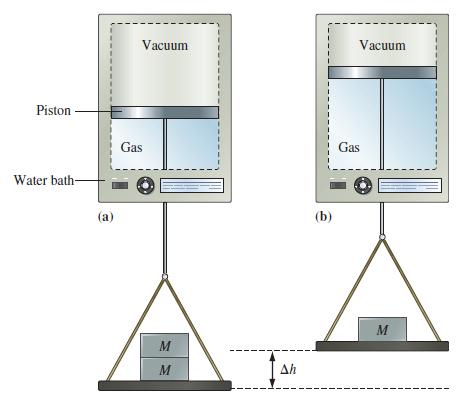
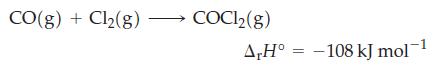
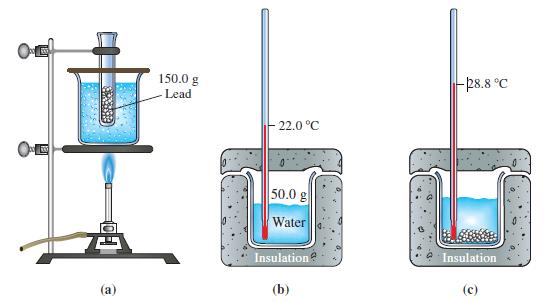
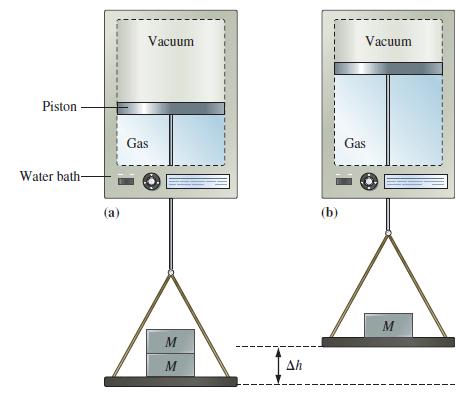
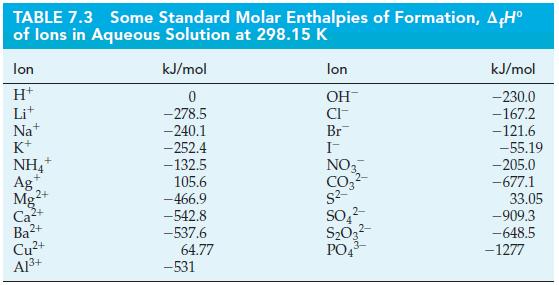
![A,H = [c x AHc + dx AHD +...] [ax AHA + bx AfHB +...] (7.22) weighted sum of A H values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/2/8/827654b39dbd60601699428827613.jpg)