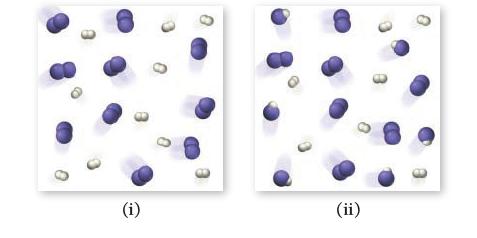
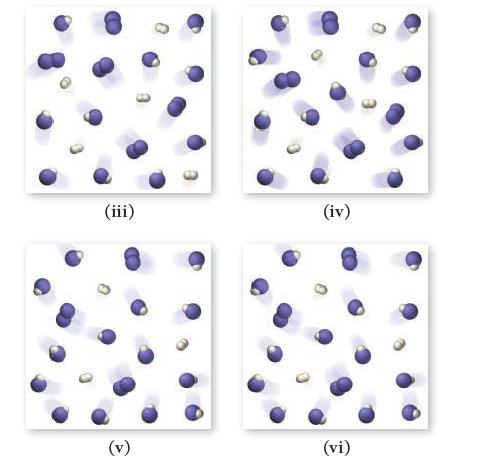
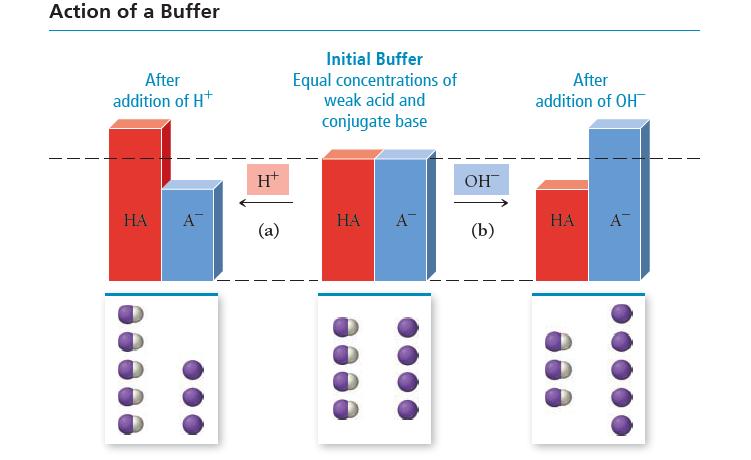
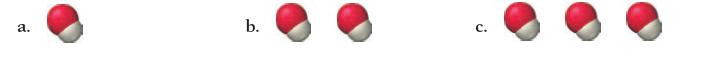![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![Concentration (M) 1.2- 1 0.8 0.6- 0.4- 0.2 [A] 0 0 10 20 30 40 50 60 70 80 Time (s)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/3446556eff8e20da1700196344748.jpg)
![[NO] (M) 0.10 0.20 0.20 0.40 [CO] (M) 0.10 0.10 0.20 0.10 Initial Rate (M/s) 0.0021 0.0082 0.0083 0.033](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/3/3/7016555fb45375d81700133699294.jpg)
![Time (s) 0 100 200 300 400 500 600 700 [SOCl] (M) 0.100 0.0971 0.0944 0.0917 0.0890 0.0865 0.0840 0.0816 Time](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/3/3/7336555fb659baa91700133731283.jpg)
![[A] (M) 0.05 0.10 0.20 [B] (M) 0.05 0.05 0.10 Initial Rate (M/s) 0.035 0.070 0.56](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/4716556f077a7e0e1700196470489.jpg)

![In [Br] -0.5- -1 -1.5 -2- -2.5+ 0 20 40 Time (s) 60 80](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/5236556f0ab2733a1700196522339.jpg)
![1/[Br] 10. 9. 8- 7 5- 4 3- 2. 1 0 0 20 40 Time (s) 60 80](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/6/5306556f0b21119e1700196529185.jpg)

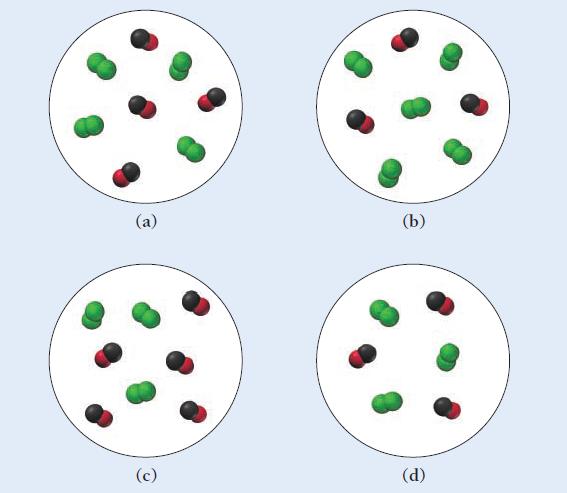
![A[Cl]/At A[F]/At -0.012 M/s A[CIF3]/At Rate](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/1136556f6e1021ae1700198110998.jpg)
![A[HS]/At A[0]/At -0.080 M/s A[HO]/At A[Ss]/At Rate](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/1526556f70862da91700198149752.jpg)
![Time (s) 0 10 20 30 40 50 [C4H8] (M) 1.000 0.913 0.835 0.763 0.697 0.637](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/4026556f802e126b1700198402474.jpg)
![Time (s) 0 10 20 30 40 50 60 70 80 90 100 [NO] (M) 1.000 0.951 0.904 0.860 0.818 0.778 0.740 0.704 0.670](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/4866556f8566bd361700198484974.jpg)
![Rate (M/s) 0.012 0.010 0.008 0.006- 0.004 0.002 0 0 0.2 0.4 0.6 [A] (M) 0.8 1](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/7786556f97af09731700198777421.jpg)
![Concentration (M) 1.2. $1.0- 0.8 0.6 0.4- 50.2 [HO] 0- 0 10 20 30 40 50 60 70 80 Time (s)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/5686556f8a897ad41700198566812.jpg)
![Rate (M/s) 0.012 0.010- 0.008 0.006 0.004 0.002 0 0 0.2 0.4 0.6 [A] (M) 0.8 1](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/7976556f98d585a31700198795517.jpg)
![[A] (M) 0.100 0.200 0.300 Initial Rate (M/s) 0.053 0.210 0.473](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/8/9586556fa2e5edc81700198958131.jpg)
![[A] (M) 0.15 0.30 0.60 Initial Rate (M/s) 0.008 0.016 0.032](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/5236556fc634ddef1700199522037.jpg)
![[A] (M) 0.12 0.16 0.20 Initial Rate (M/s) 0.0078 0.0104 0.0130](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/5396556fc73852831700199538160.jpg)
![[A] (M) 0.12 0.18 0.28 Initial Rate (M/s) 3.89 x 10-4 8.75 X 10-4 2.12 x 10-3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/7056556fd19bf2df1700199704049.jpg)
![[NO] 0.100 0.200 0.200 0.400 [F] (M) 0.100 0.100 0.200 0.400 Initial Rate (M/s) 0.026 0.051 0.103 0.411](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/7286556fd309b4941700199727229.jpg)
![CH3CI(g) [CH3CI] (M) 0.050 0.100 0.100 0.200 + 3 Cl(g) [Cl] (M) 0.050 0.050 0.100 0.200 CCl4(g) + 3 HCl(g)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/7506556fd46e3c351700199748951.jpg)
![AB(g) Time (s) 0 50 100 150 200 250 300 350 400 450 500 A(g) + B(g) [AB] (M) 0.950 0.459 0.302 0.225 0.180](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/7866556fd6ab86171700199784137.jpg)
![NO5 (g) Time (s) 0 25 50 75 100 125 150 175 200 NO3(g) + NO(8) [NO5] (M) 1.000 0.822 0.677 0.557 0.458 0.377](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/8116556fd83eda1f1700199809723.jpg)
![C4H8 Time (s) 0 10 20 30 40 50 60 70 80 90 100 2 CH4 [C4H8] (M) 1.000 0.894 0.799 0.714 0.638 0.571 0.510](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/8986556fdda6b91f1700199896318.jpg)
![Time (s) 0 25 50 75 100 125 150 175 200 [A] (M) 1.000 0.914 0.829 0.744 0.659 0.573 0.488 0.403 0.318](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/9/9/9326556fdfccab481700199930382.jpg)