![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![Rate = k[A] (first-order rate law) d[A] Rate = dt d[A] dt -k[A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/4/13565570e67ab0db1700204132464.jpg)
![d[A] [A] IA]d[A] [A]o [A] -kdt -S kdt](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/4/15965570e7f50ae81700204156168.jpg)
![[In[A]] = k[t] In[A] In[A]o -kt In[A] = -kt + In[A]o (integrated rate law)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/4/18365570e97069641700204178442.jpg)
 0 0.193 t (s) 0 884 0.316 0.427 0.784 1610 2460 50,000](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/4/35665570f448efea1700204352677.jpg)
![Time (s) 0 10 20 30 40 50 60 25.0 C [A] (M) 1.000 0.779 0.591 0.453 0.338 0.259 0.200 35.0 C [A] (M) 1.000](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/5/688655714788b8921700205687143.jpg)
![Time (s) 0 0.1 0.2 0.3 0.4 0.5 0.6 25.0 C [A] (M) 1.000 0.724 0.511 0.375 0.275 0.198 0.141 35.0 C [A] (M)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/5/7106557148eaedb41700205708921.jpg)
![Initial Rate vs. Initial [CH4] 0.010 0.020 0.020 [03] 0.010 0.010 0.020 Concentrations Initial Rate (M/s)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/5/774655714ceb5f7b1700205773653.jpg)

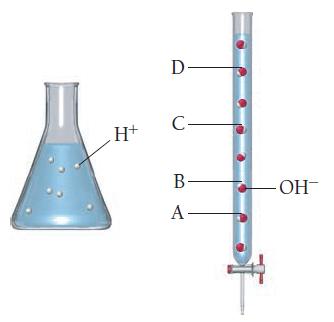
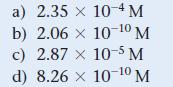
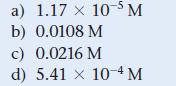
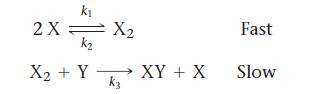
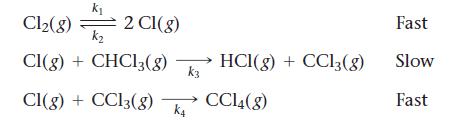
![CH3CN(g) Time (h) 0.0 5.0 10.0 15.0 20.0 25.0 CH3NC(g) [CH3CN] (M) 1.000 0.794 0.631 0.501 0.398 0.316](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/1/440655703e06d9341700201438304.jpg)
![XY Time (h) 0.0 1.0 2.0 3.0 4.0 5.0 2X + Y [XY] (M) 0.100 0.0856 0.0748 0.0664 0.0598 0.0543](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/1/472655704008ff6b1700201470675.jpg)
![[A][C] [B]/2 Rate = k](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/1/633655704a1e35021700201632477.jpg)