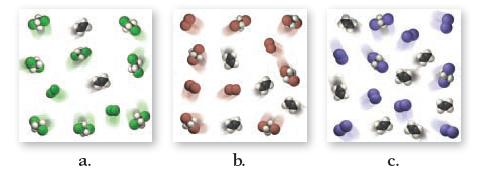
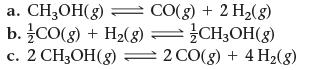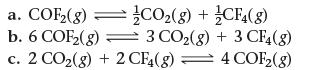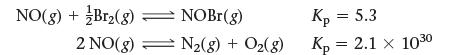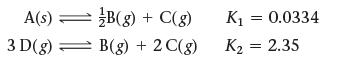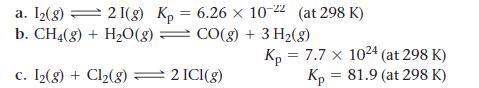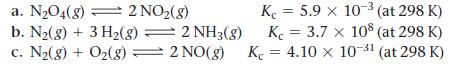![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


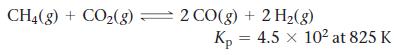
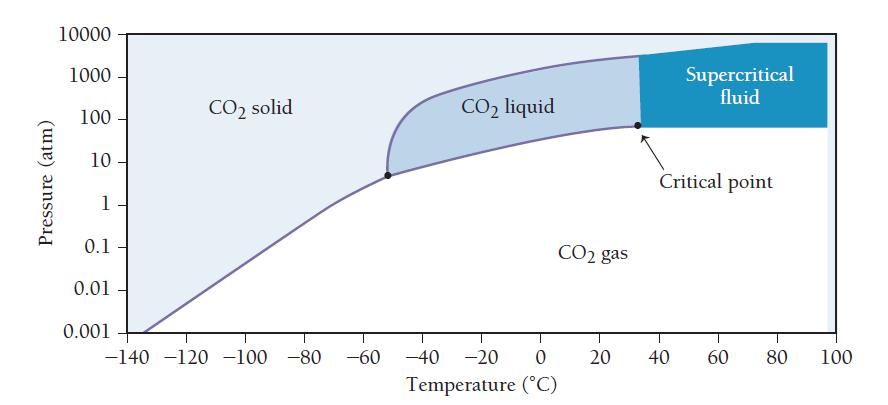
![Concentration 1.0 M- 0.5 M [A] [B] Time-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/1/8/93765574839d375f1700218936461.jpg)
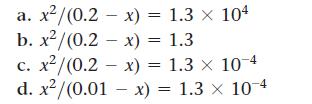
![a) Ke c) Ke = [CH] [H] [CH] [C][H1 b) K d) Ke = [CH] [C] [H] [CH] [H1](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/1/2/07665572d6ce69fe1700212077293.jpg)
![(a) K = [C] [A] [B] (b) K = 3 [C] 2 [A] [B] (c) K = [A] [B] [C] (d) K = [C] [A] [B]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/1/1/35565572a9be07301700211356456.jpg)

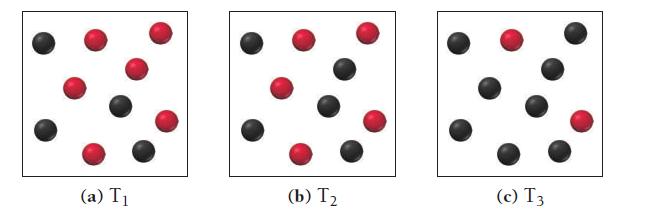
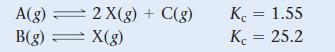

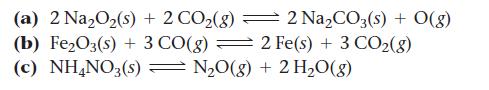
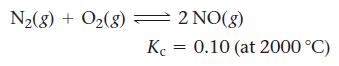
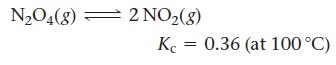
![(a) K = 1.0 x 105; [A] = 0.250 M (c) K = 1.0 10-5; [A] = 0.00250 M (b) K = 1.0 10-2; [A] (d) K = 1.0 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/1/1/89265572cb4a748f1700211892693.jpg)
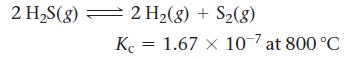
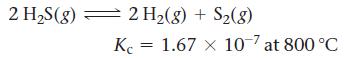
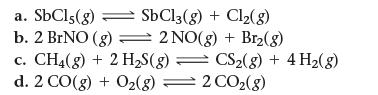
![a. 2 HS(g) 2 H(g) + S(g) K = b. CO(g) + Cl(g) = COC12(g) K = [H][S] [HS] [CO][C1] [COCl2]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/1/2/959655730df43cbd1700212958508.jpg)