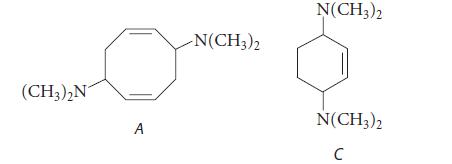
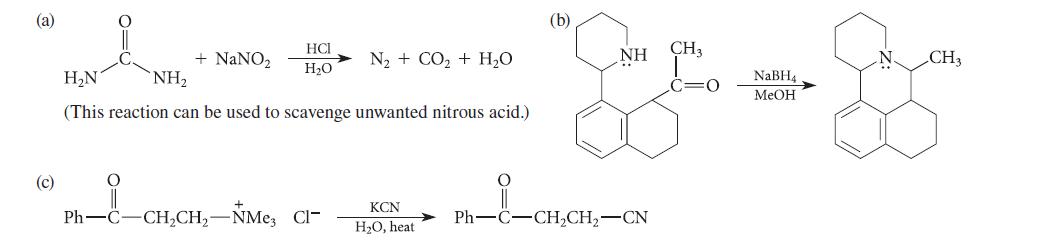
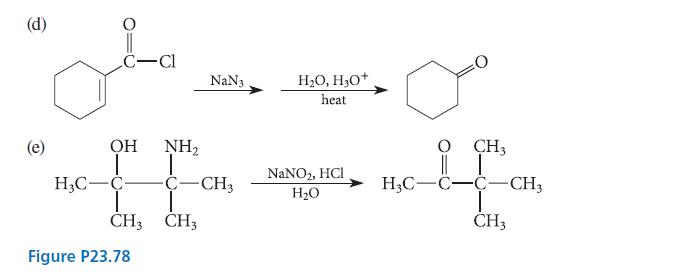
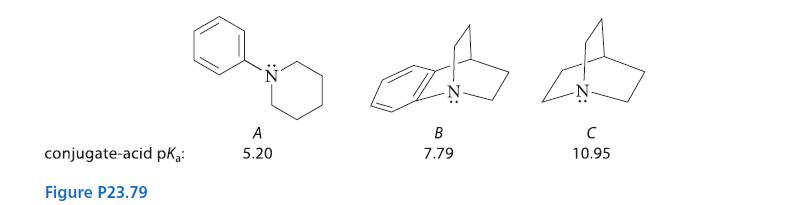
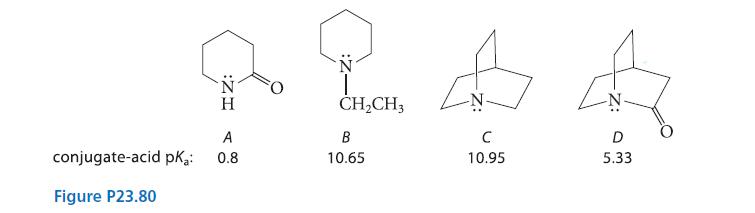
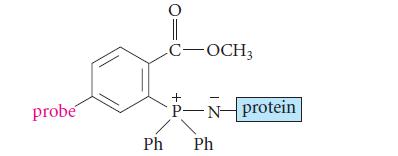
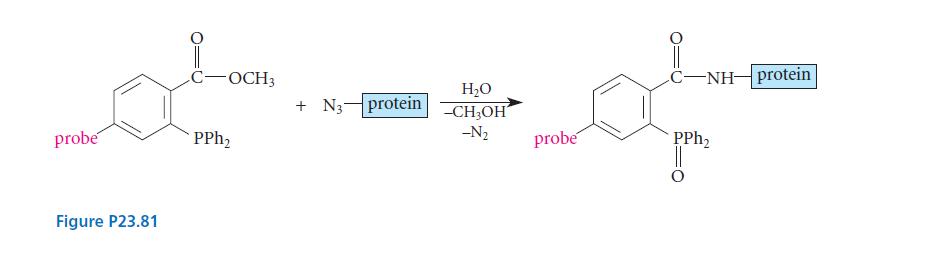
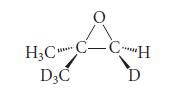
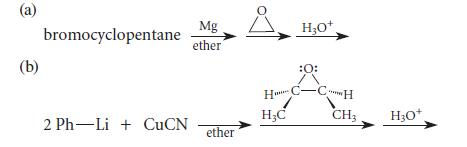
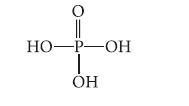
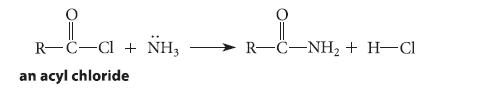
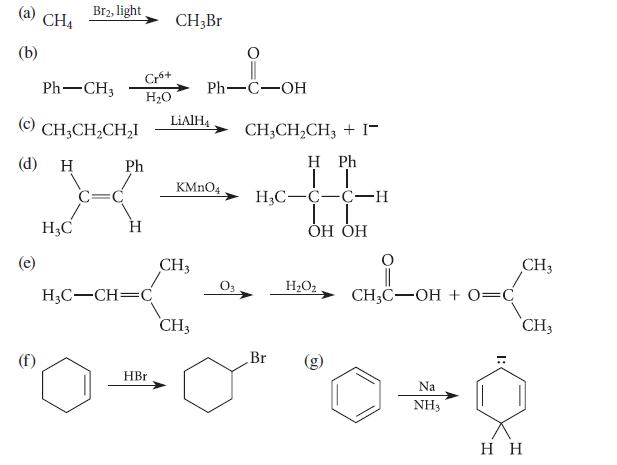
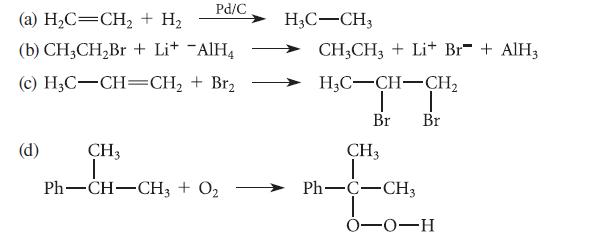
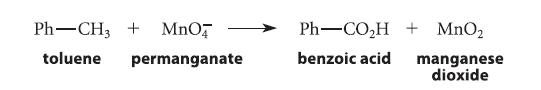
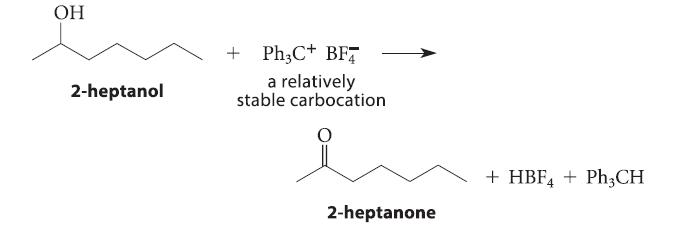
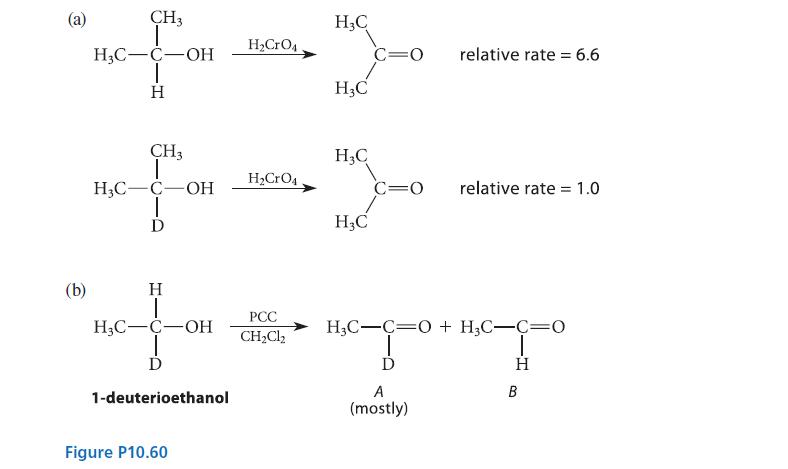
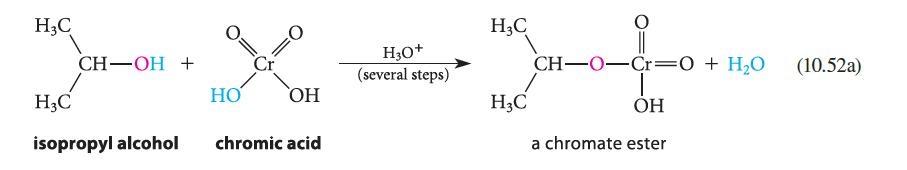
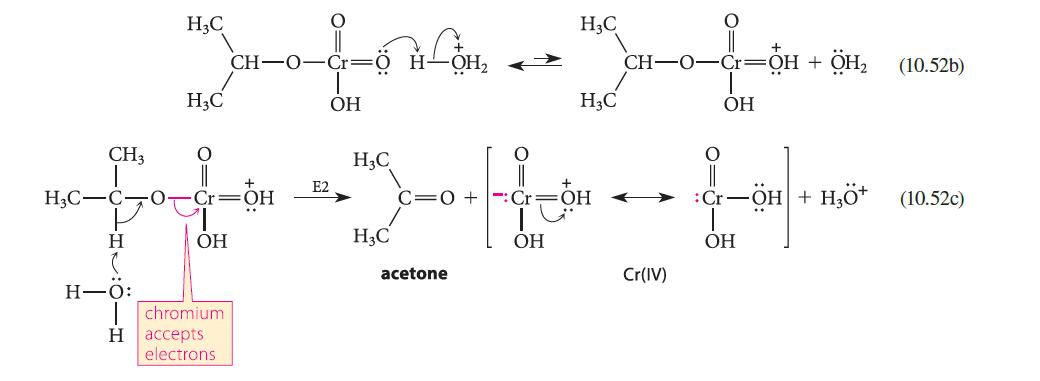
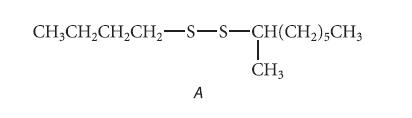
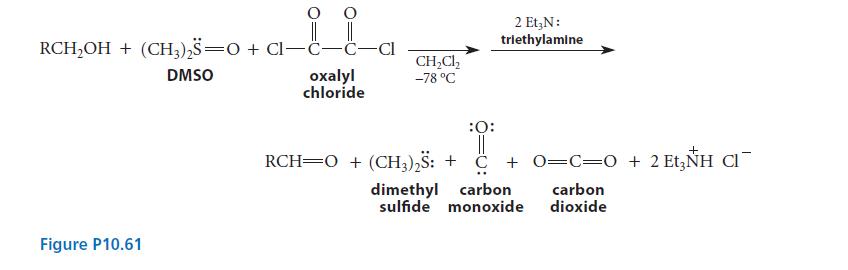
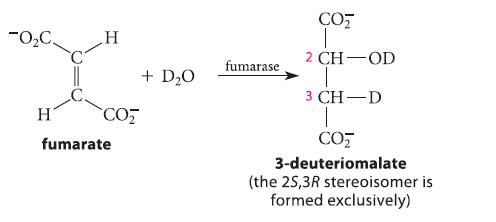
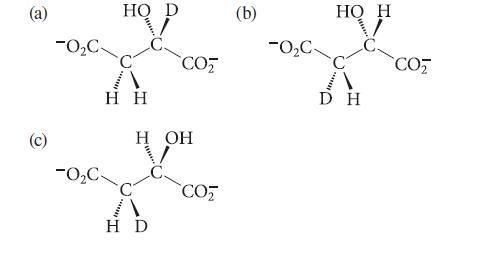
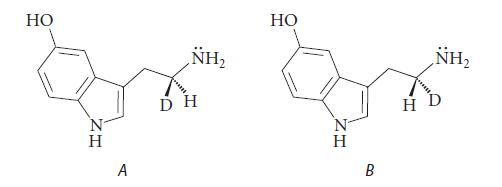
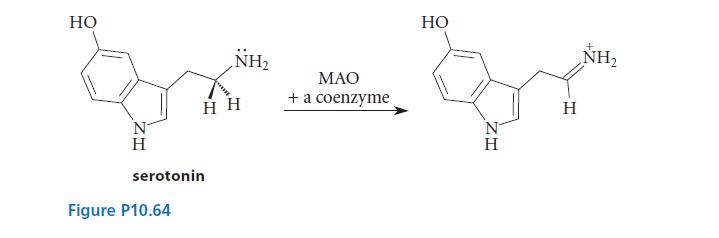
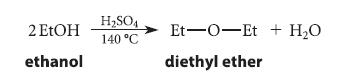
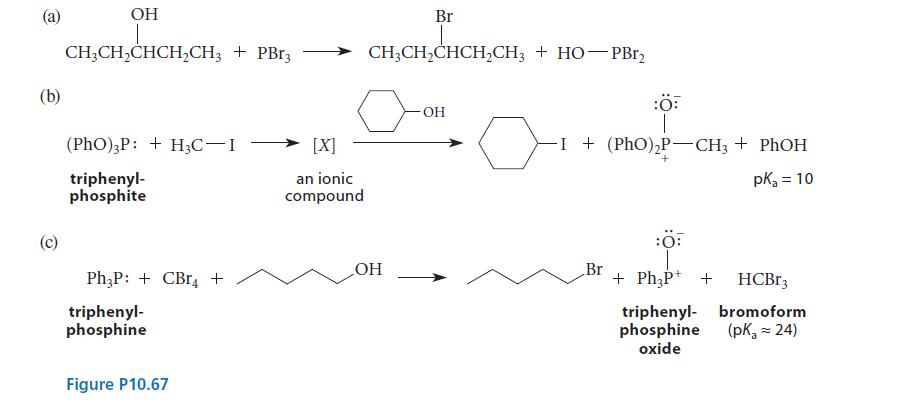
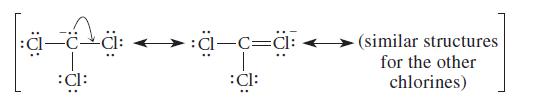
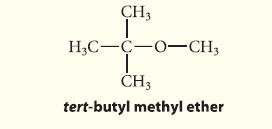
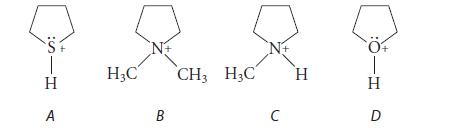
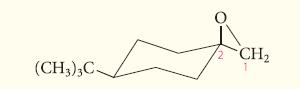
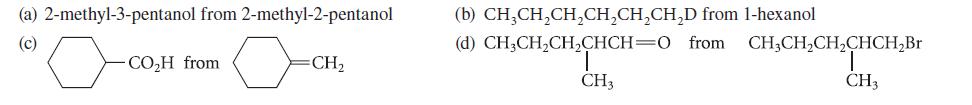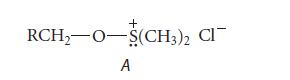![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
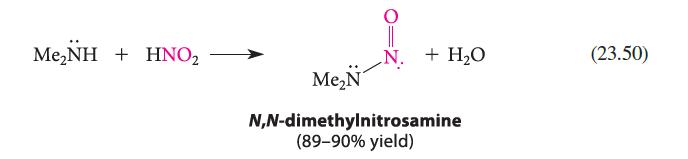
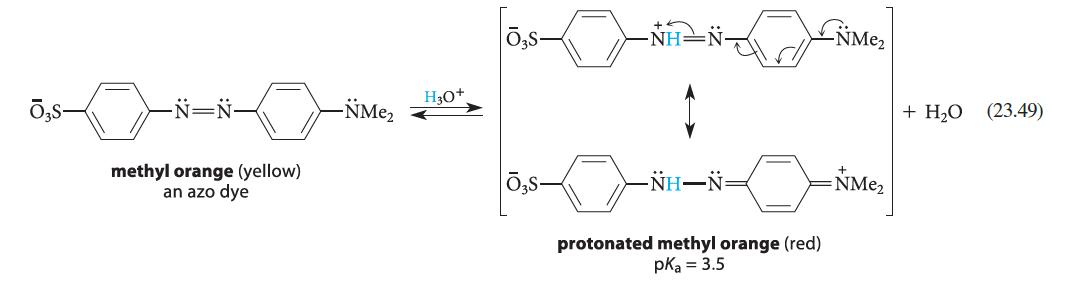
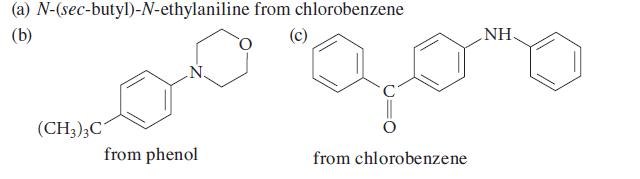
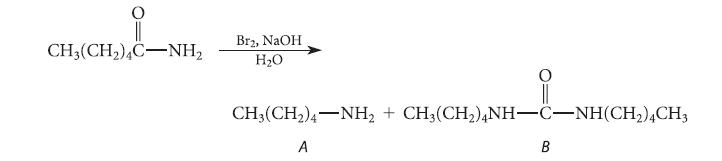
![]()


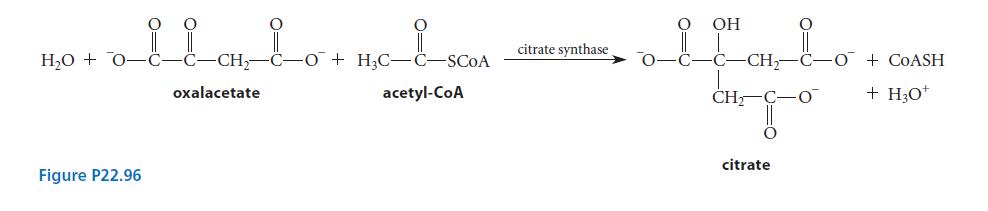
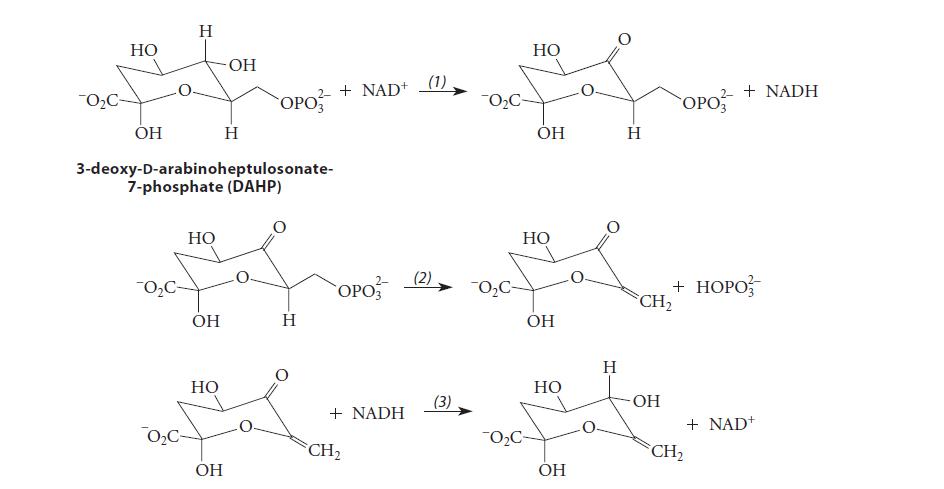
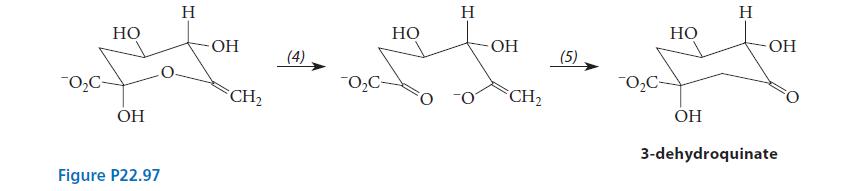
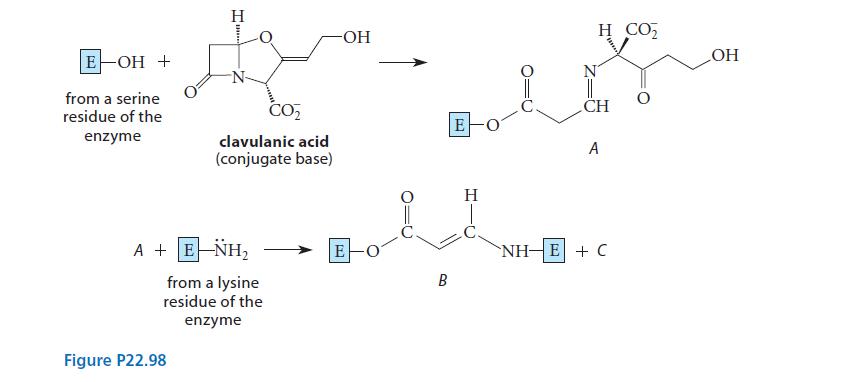
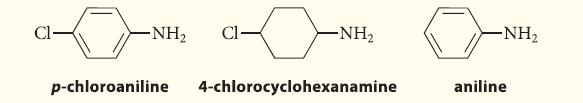
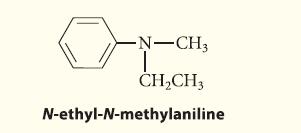
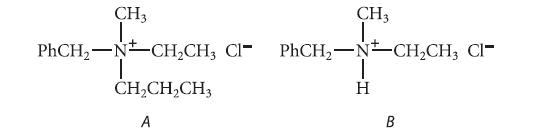
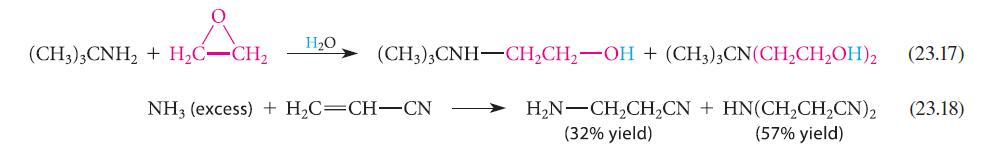
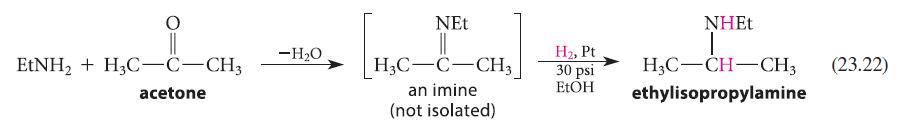
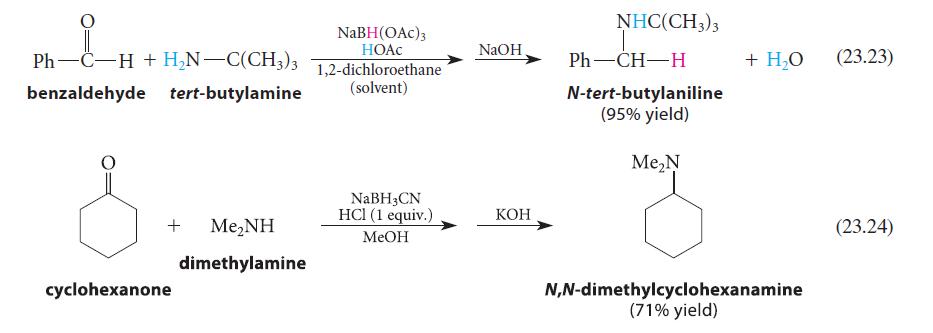
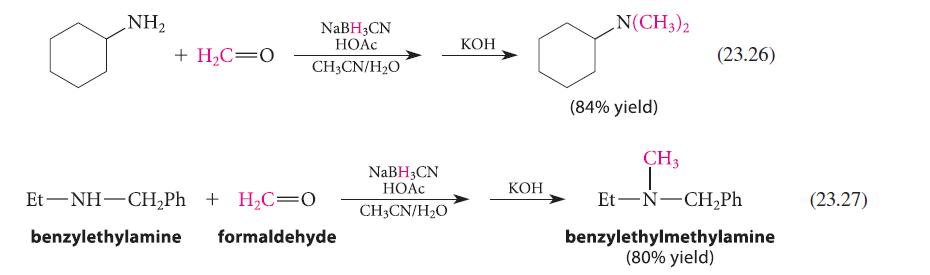
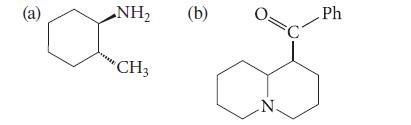
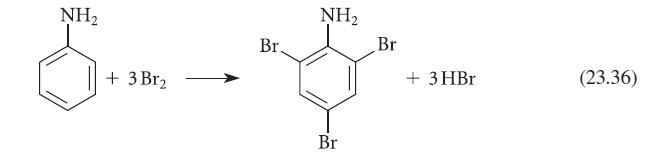
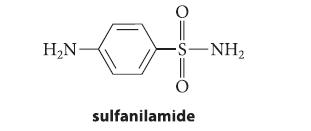
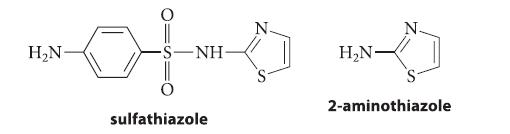
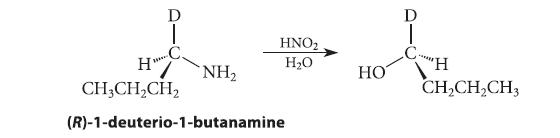
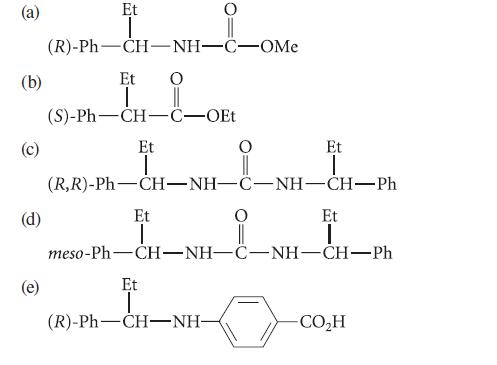
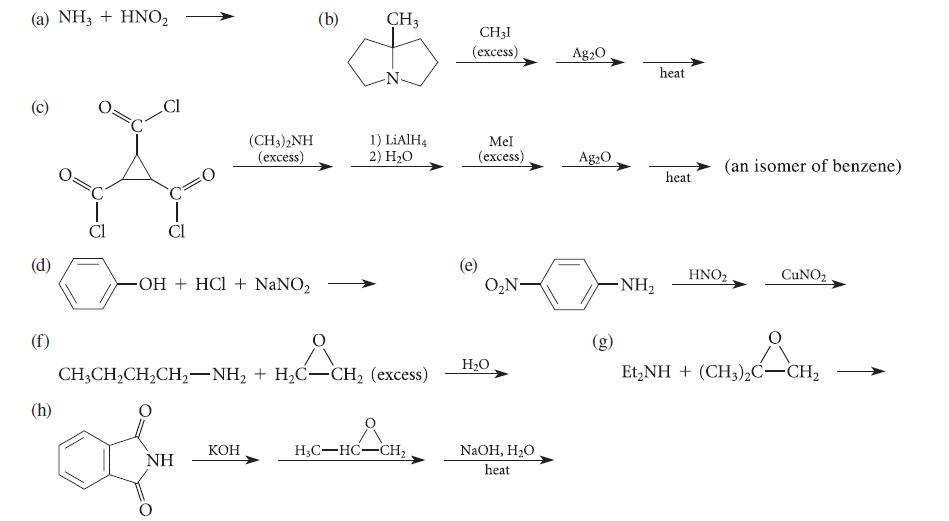
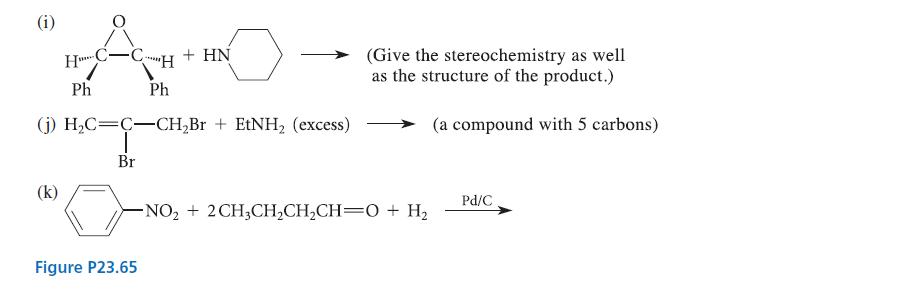
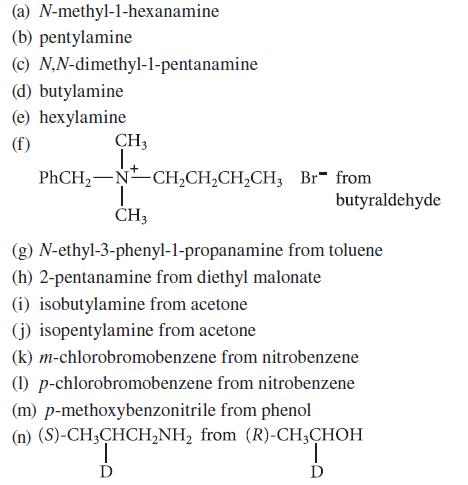
![CH3(CH)3CH=0+ NH3 + H Figure P23.76 catalyst CH3(CH)3CHNH + [CH3(CH)3CH)2NH + [CH3(CH)3CH] 3N](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/3/0/475657165ebce3f81701930474798.jpg)