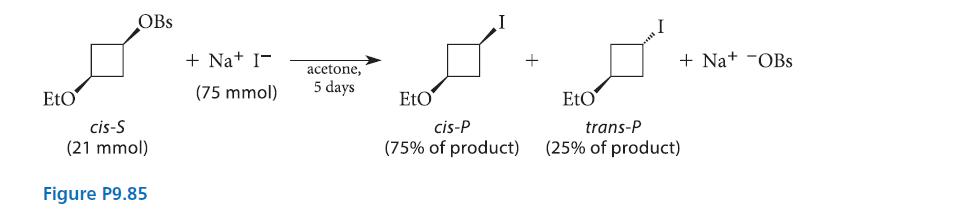
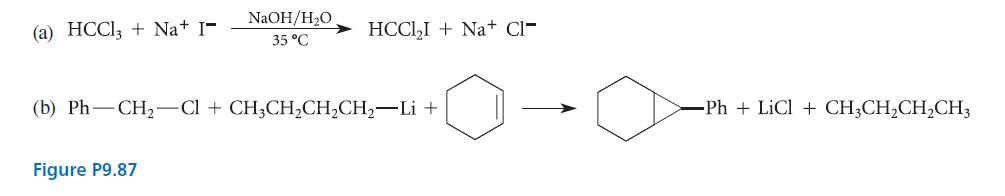
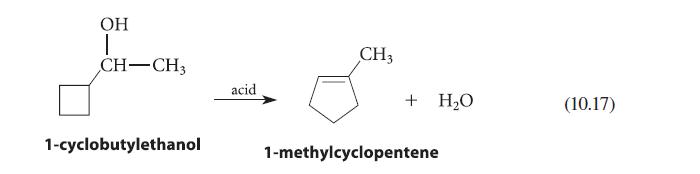
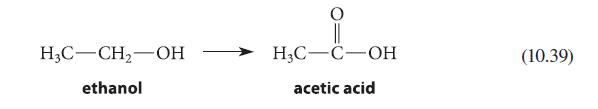
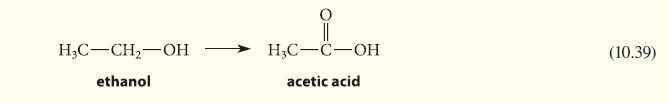
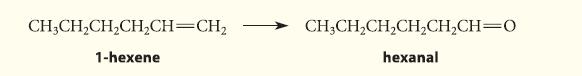
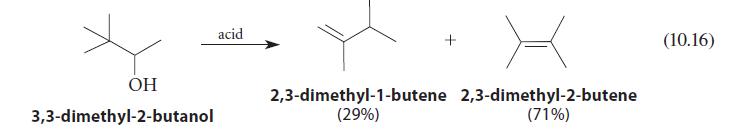
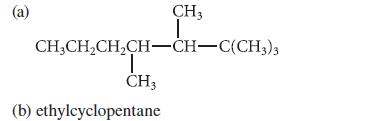
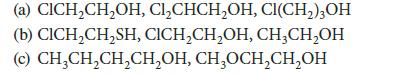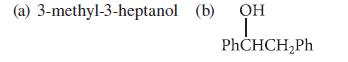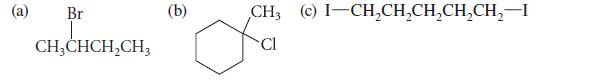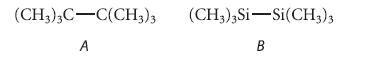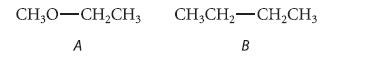![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


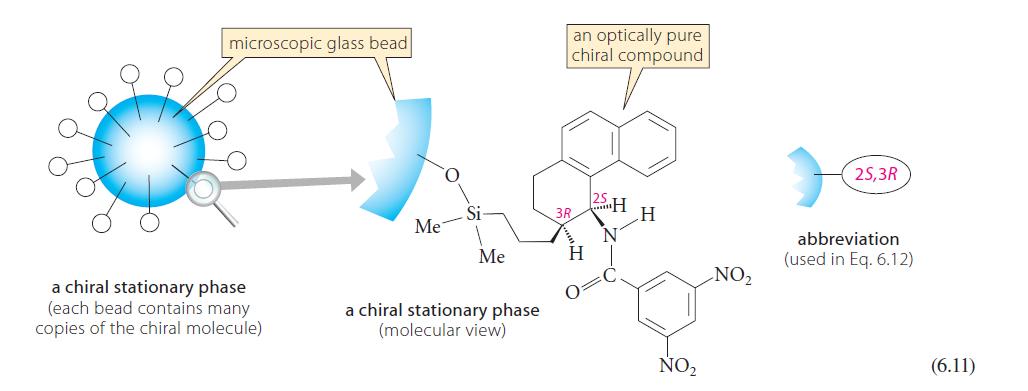
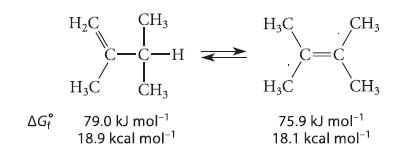
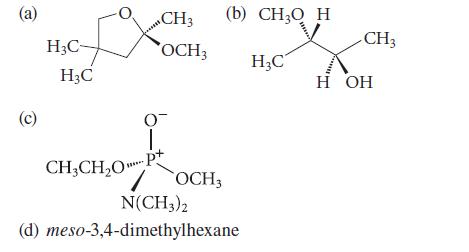
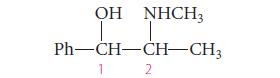
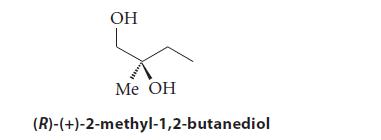
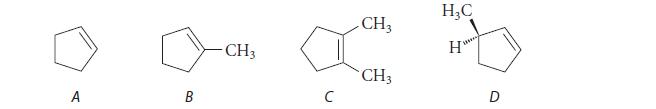
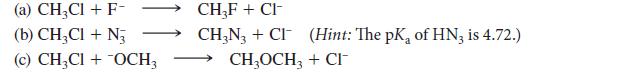
![Lo HCC=O-H+:NH, acetic acid HCCO +NH, 3 || rate = K[HC-C-OH][NH]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/4/0/6366566df3c2e8c21701240635245.jpg)
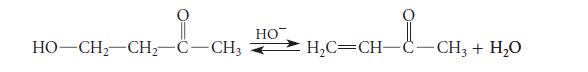
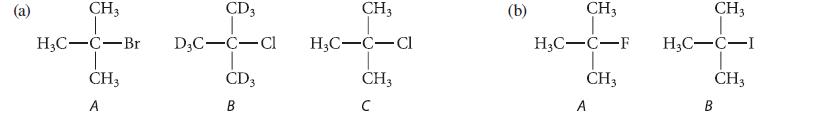
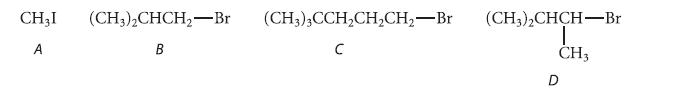
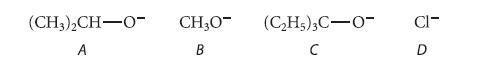
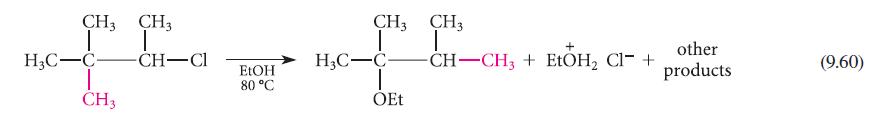
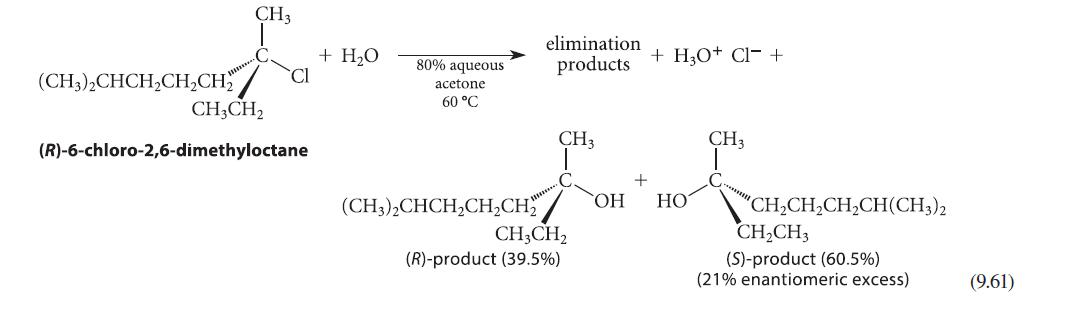
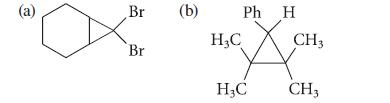
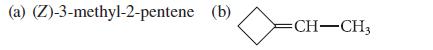
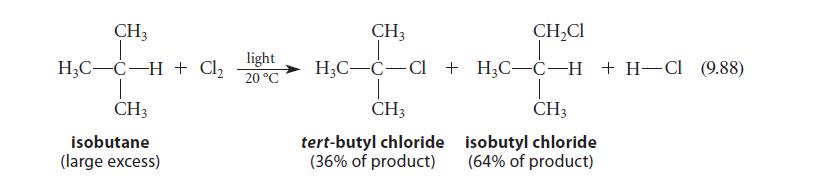
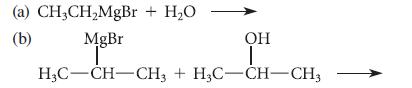
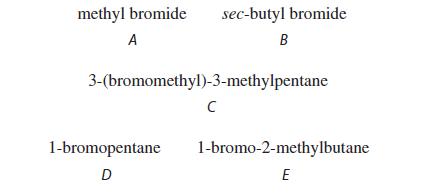
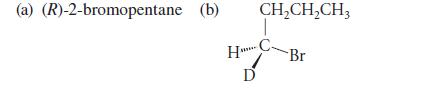

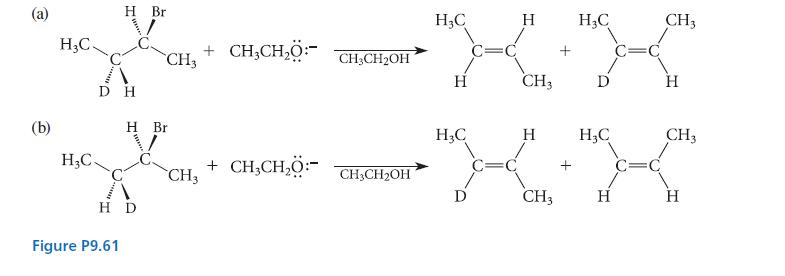
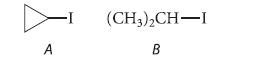
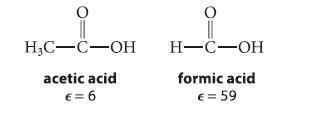
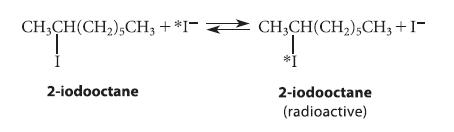
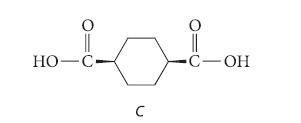
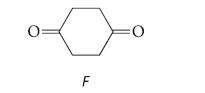
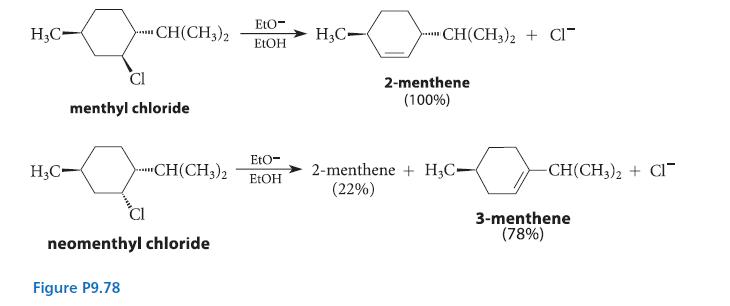
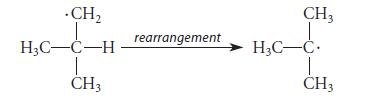
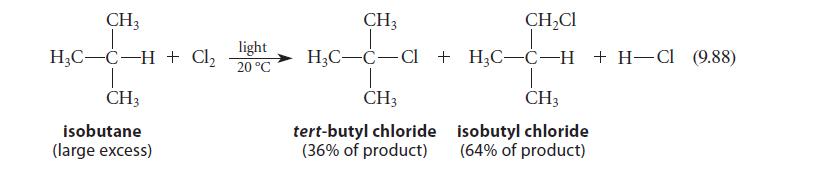
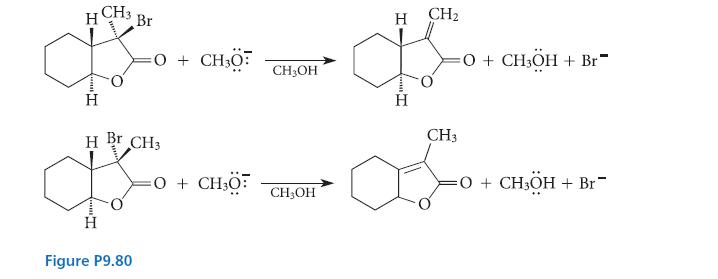
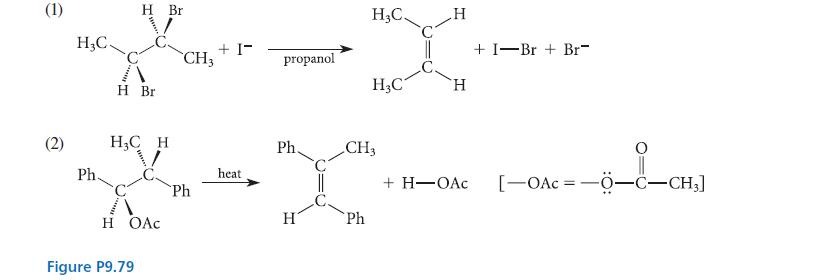
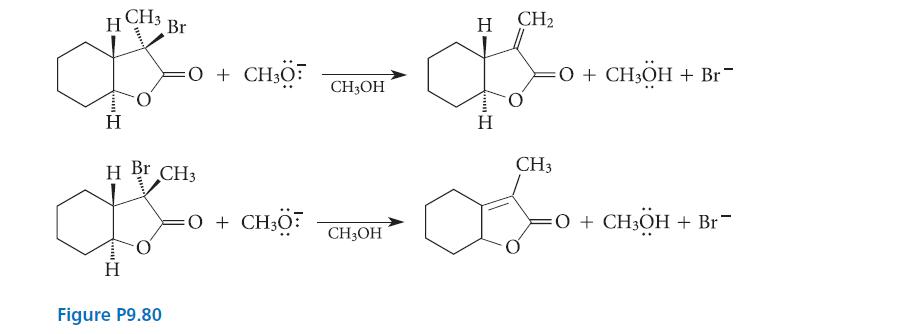
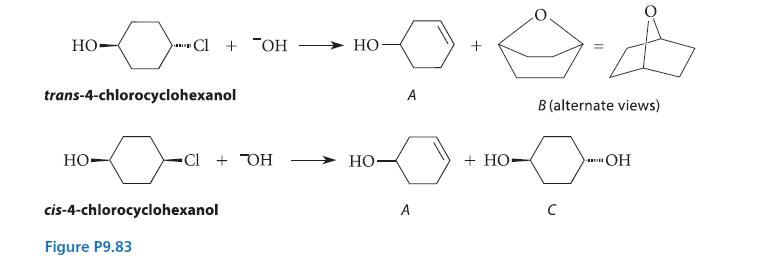
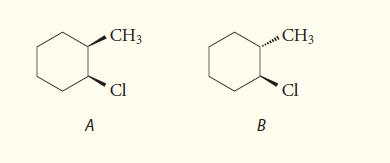
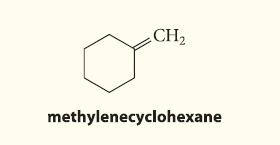
![NH -Br A + NH Br rate = k[A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/5/0/727656706a77a7eb1701250725567.jpg)