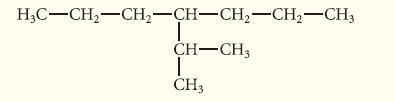
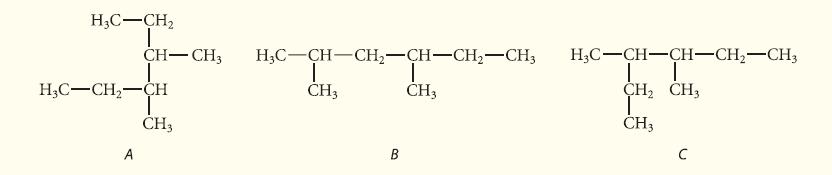
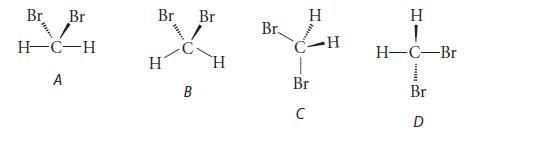
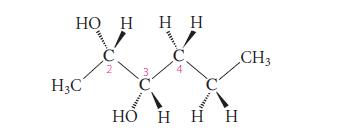
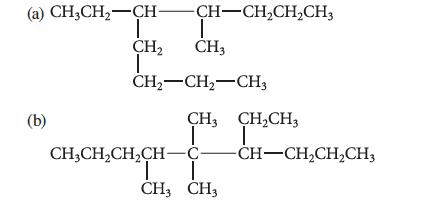
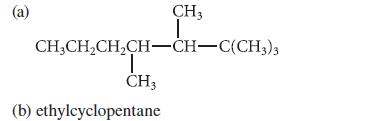
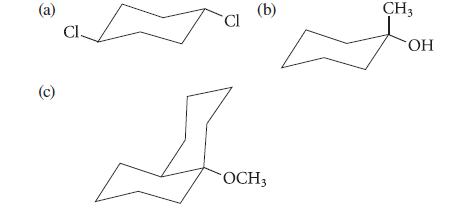
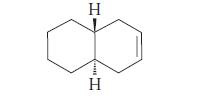
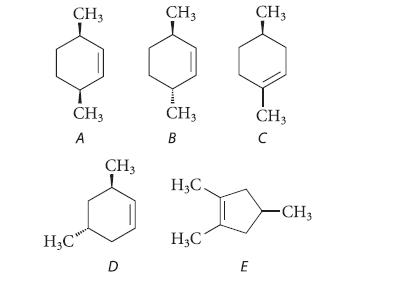
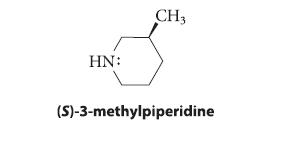
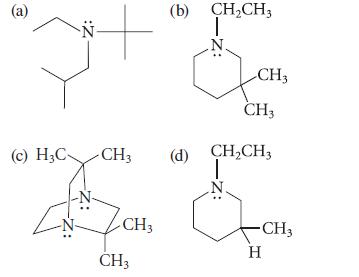
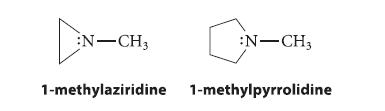
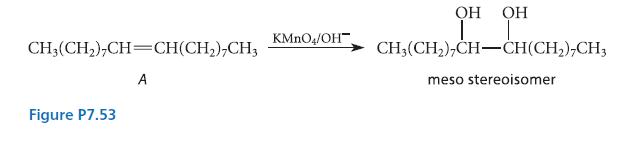
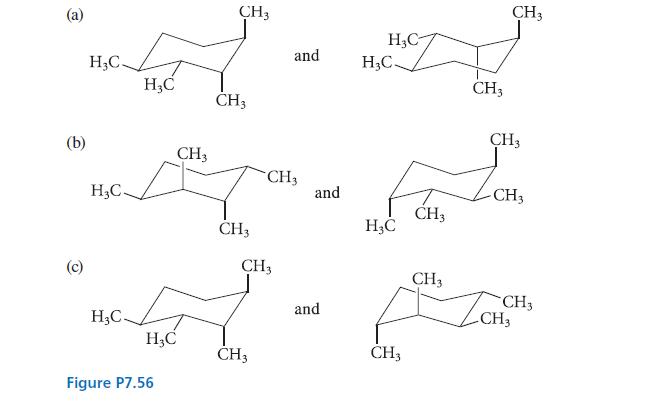
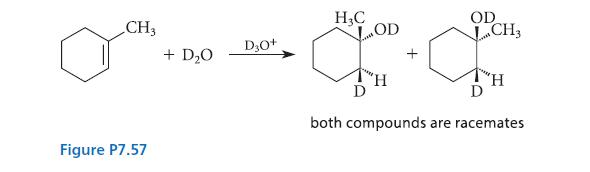
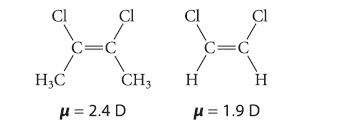
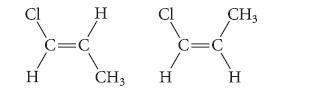
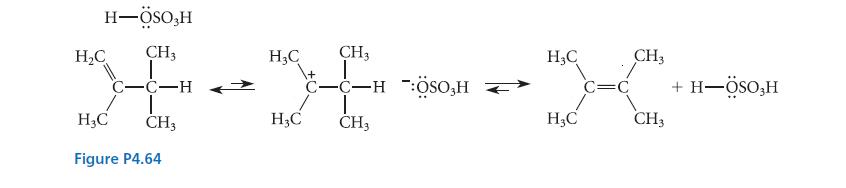
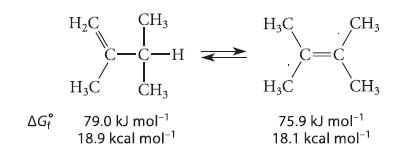
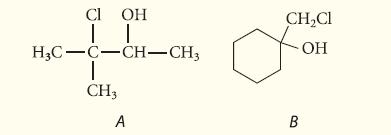
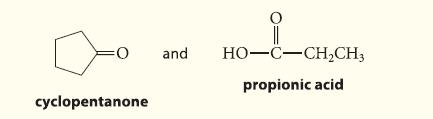
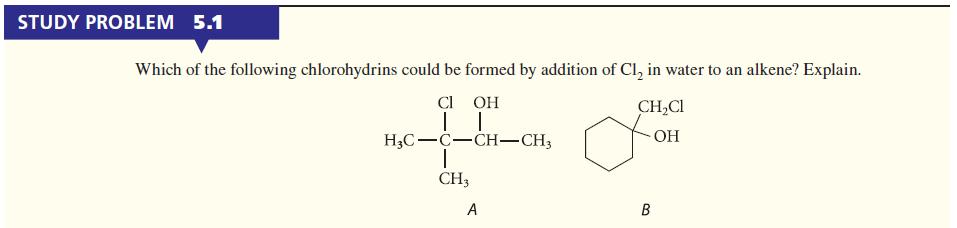
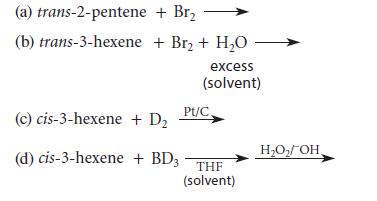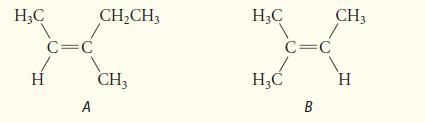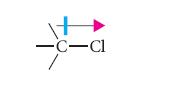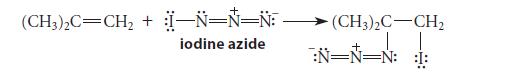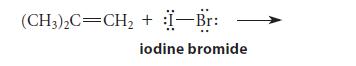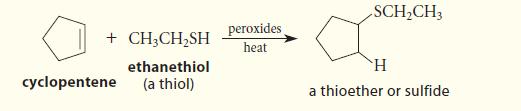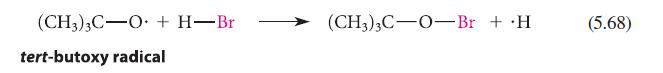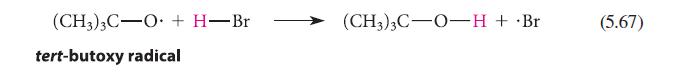![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


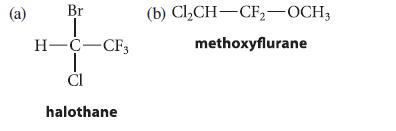
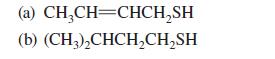
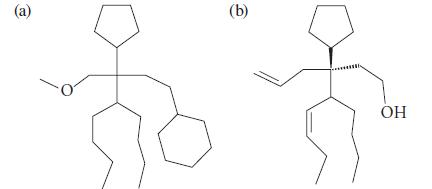

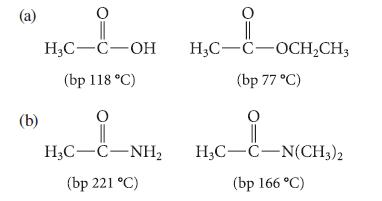
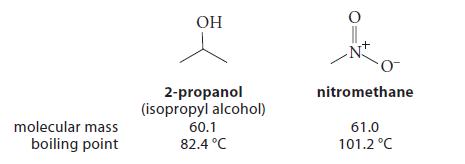
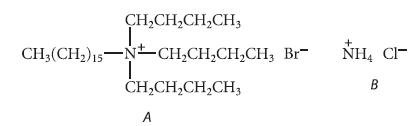
![K = [crown ether][M*] [crown ether-M* complex]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/3/6/4156566cebf186c31701236414288.jpg)
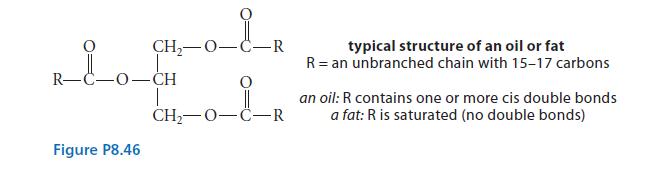
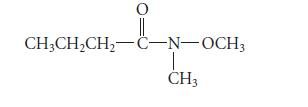
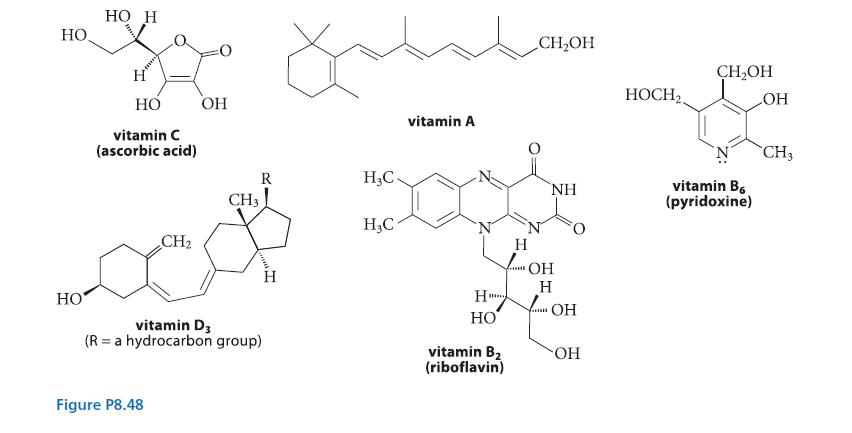

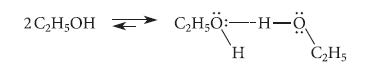
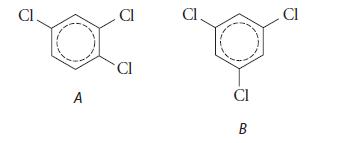
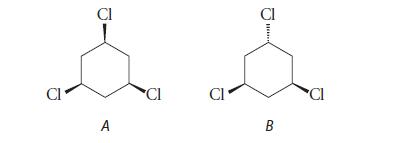
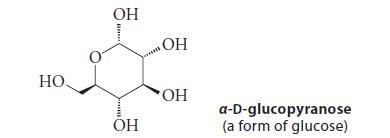

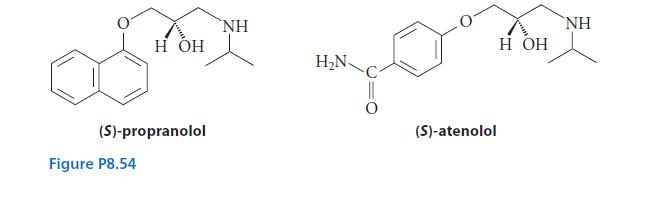
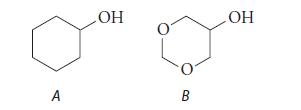
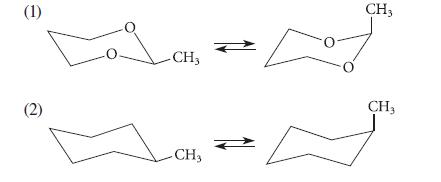
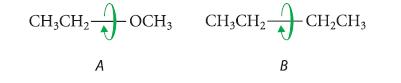
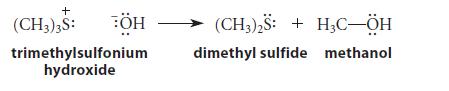
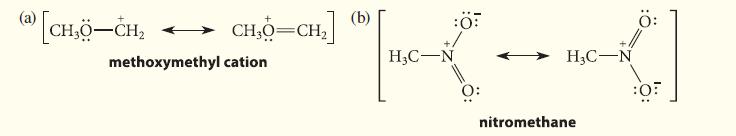
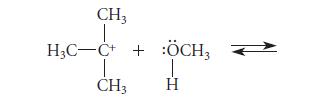
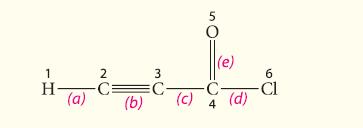
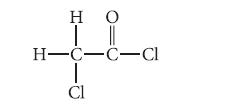
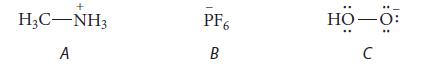
![HC=CH-CH allyl anion ]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/1/6/770655ee0e2bb2c81700716770765.jpg)
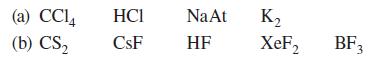
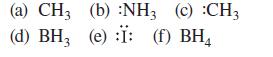
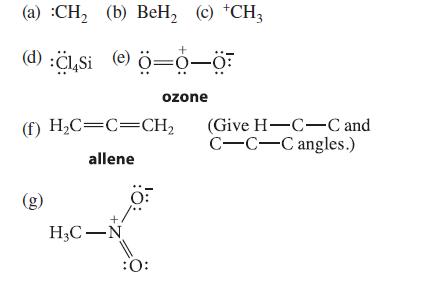
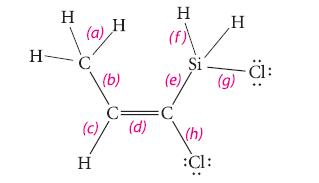
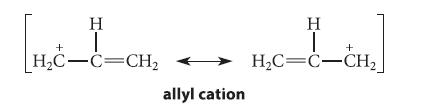

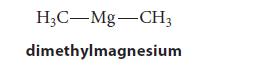
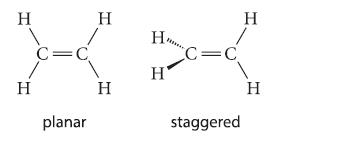
![[] HC-N :O: HC-N :O: \.. (1.6)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/2/425655ef6f90a04a1700722419352.jpg)