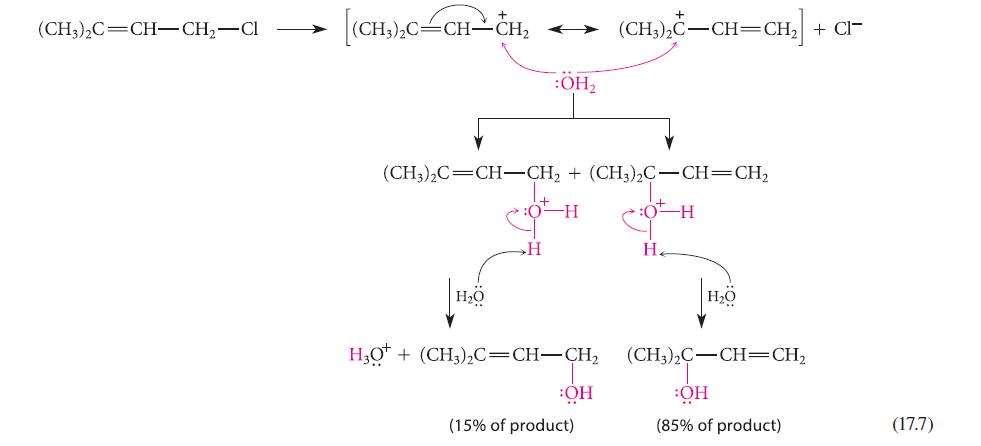
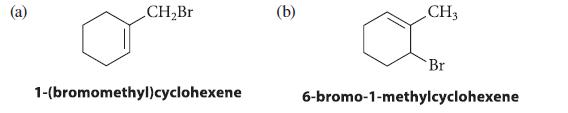
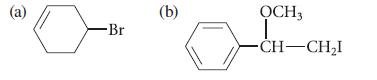
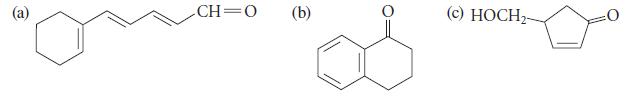
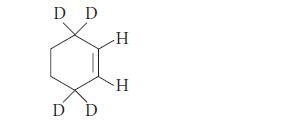
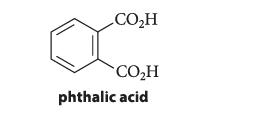
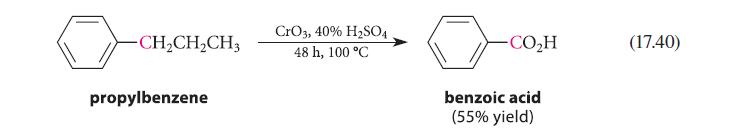
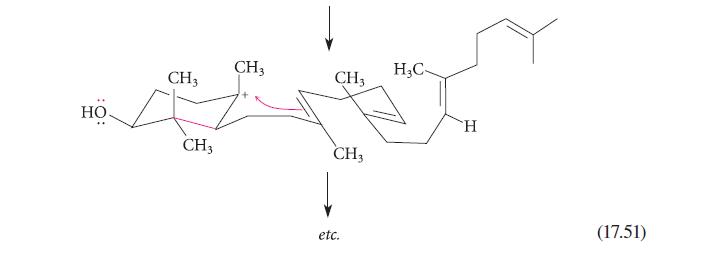
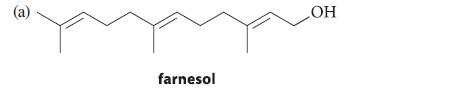
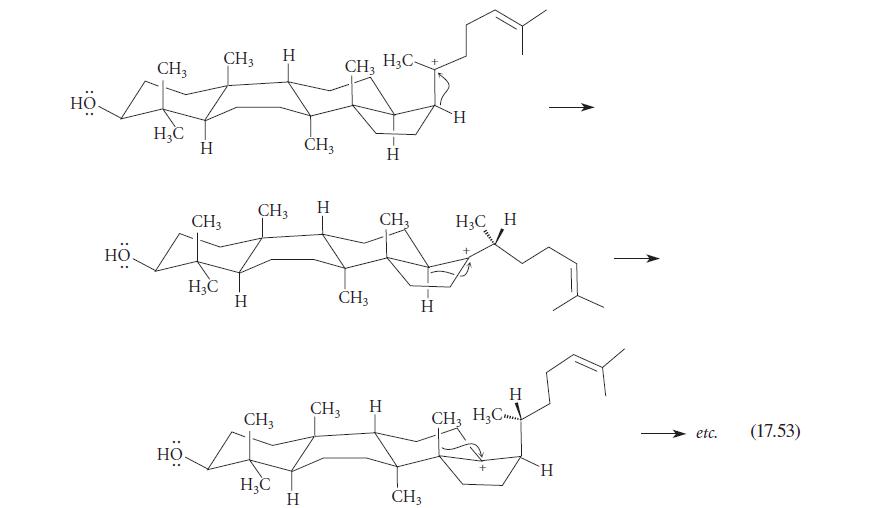
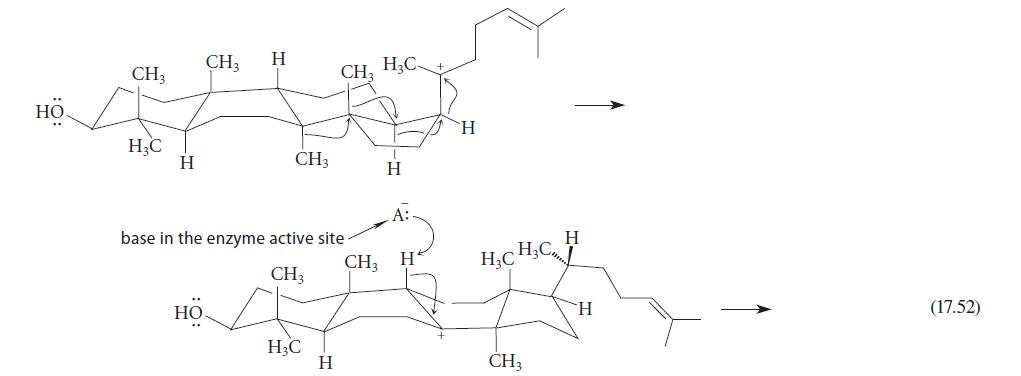
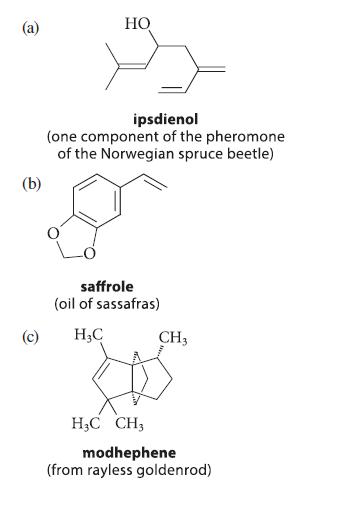
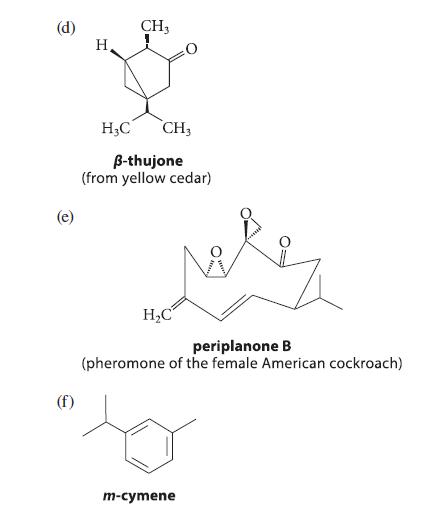
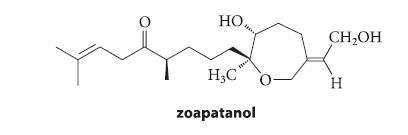
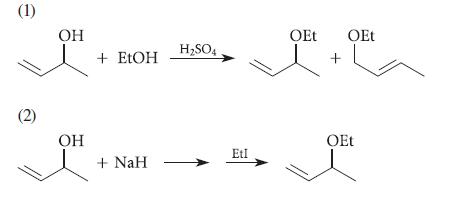
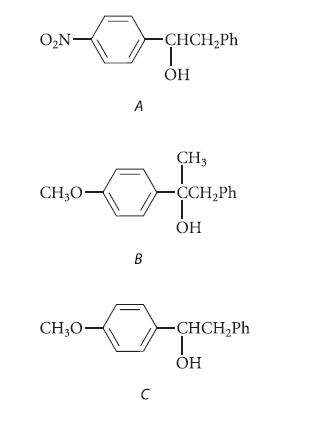
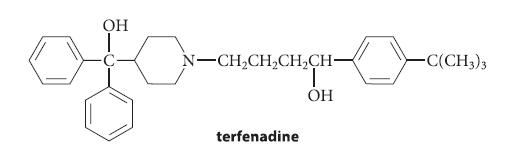
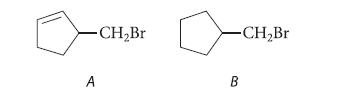
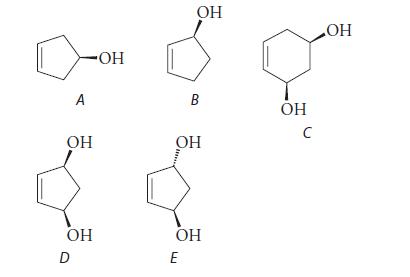
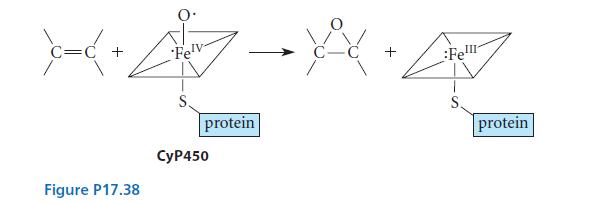
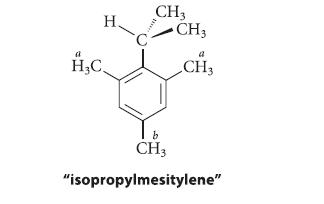
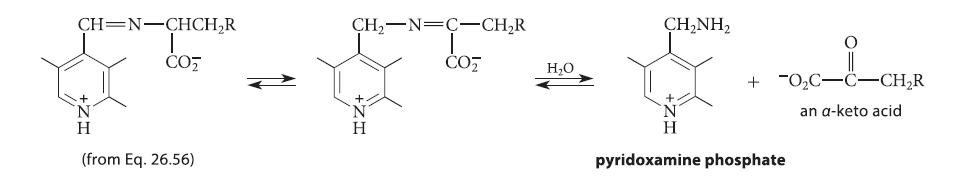
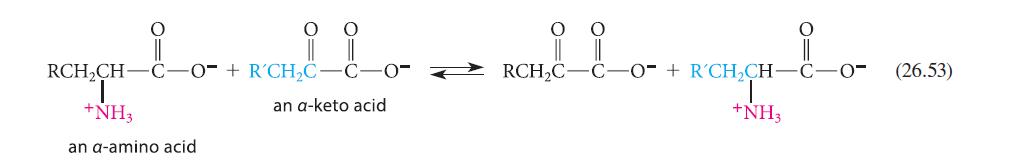
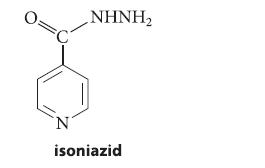
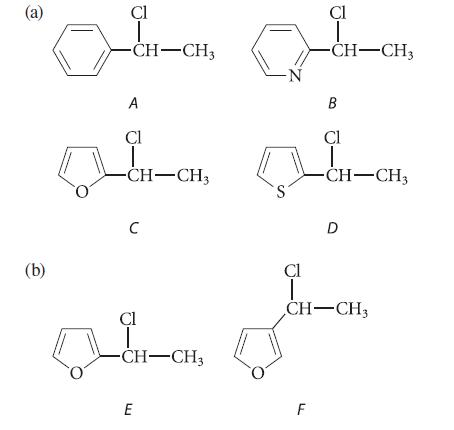
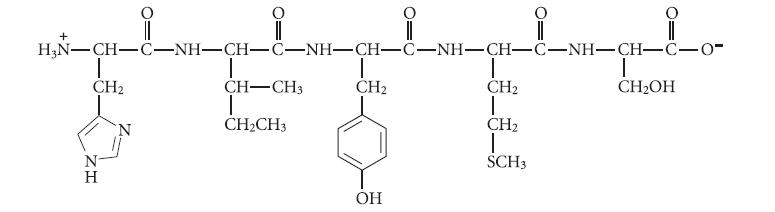
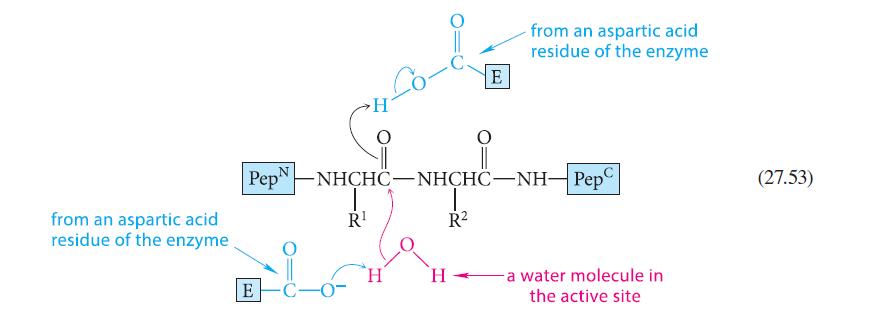
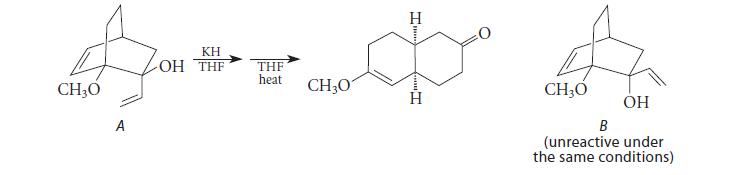
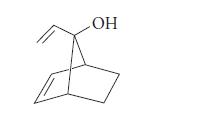
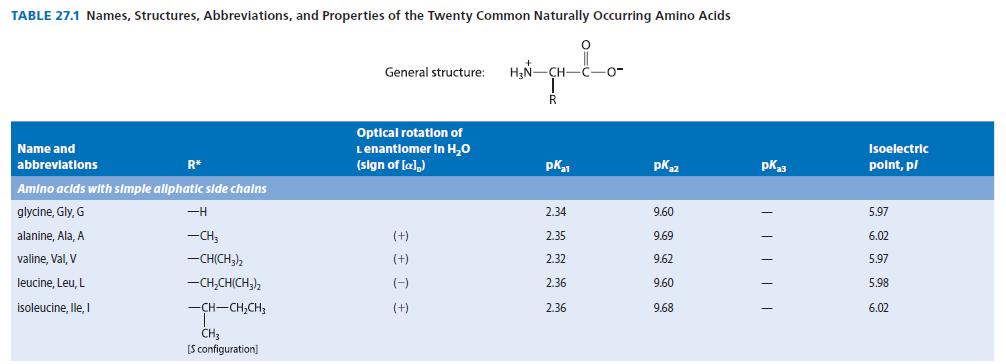
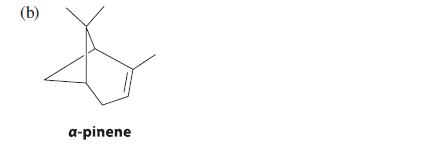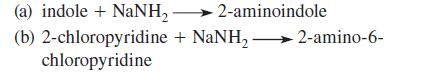![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


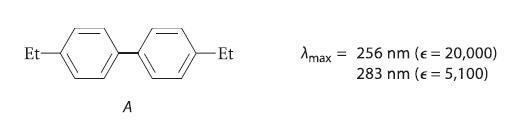
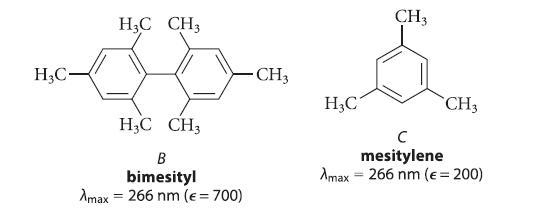
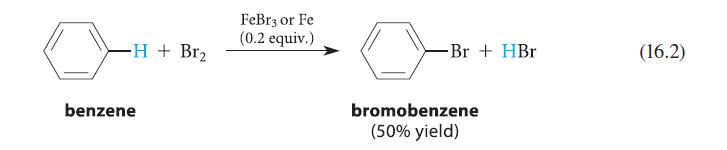
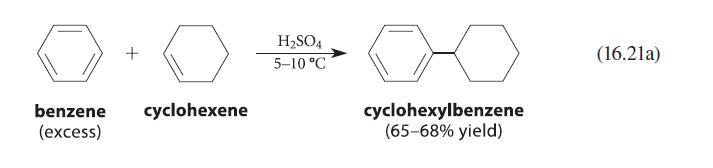
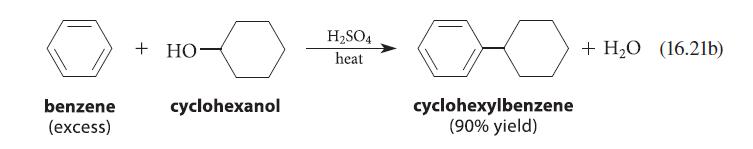
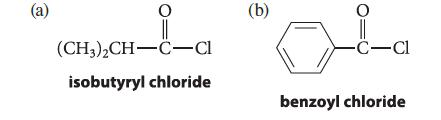
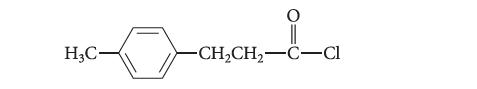
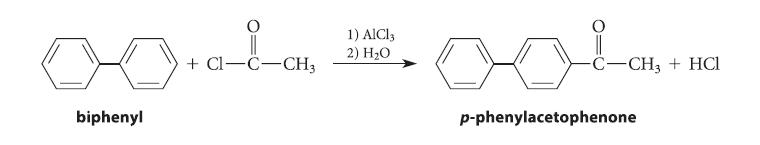
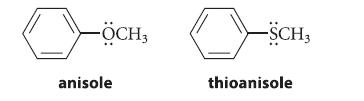
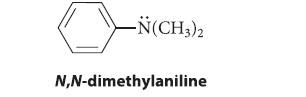
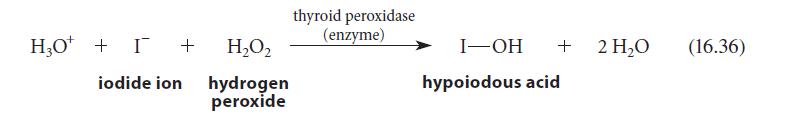
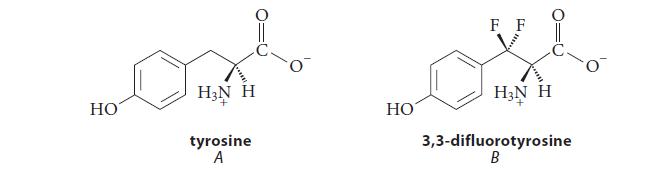
![benzo[a]pyrene living tissue (enzymes, O) HO" OH benzo[a]pyrene diol-epoxide (16.50)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/6/6/6/340656d5e24769351701666338943.jpg)
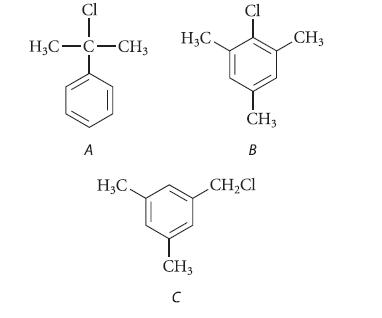
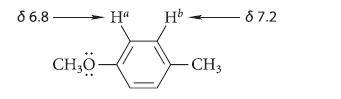
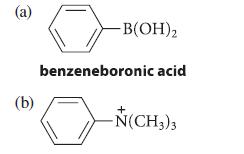
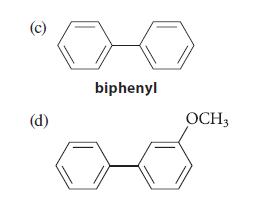
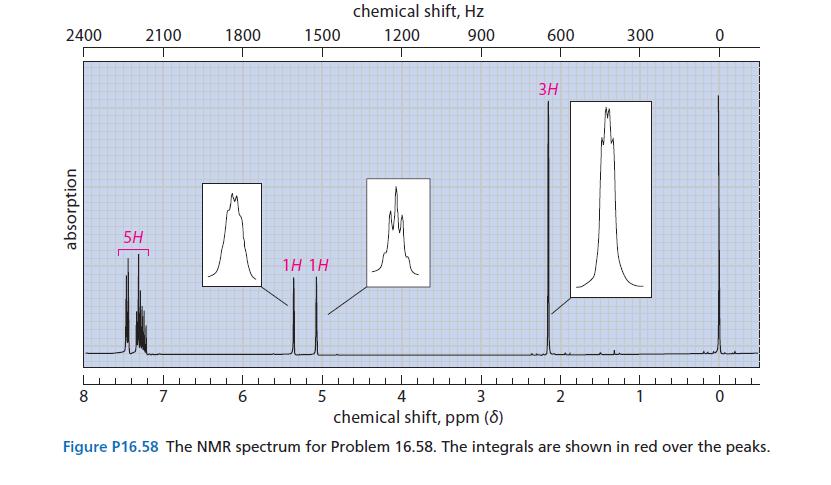
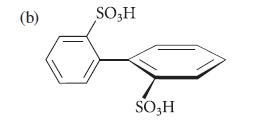
![2 -CH=CH styrene Figure P16.56 RSOH RSO3H [X] (C16H16) Y CH3 Ph N CH3 Ph](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/6/6/7/208656d6188f0a891701667207159.jpg)
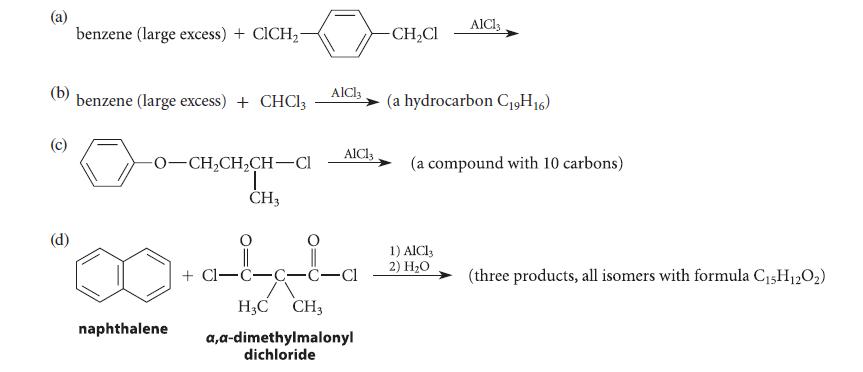
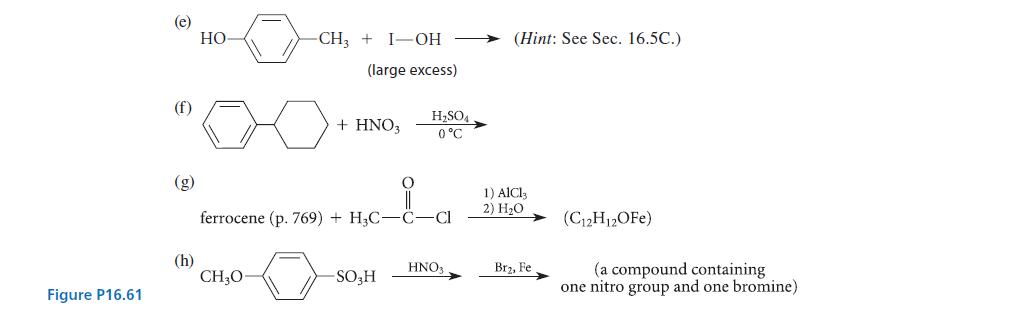
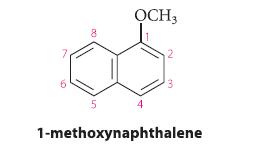
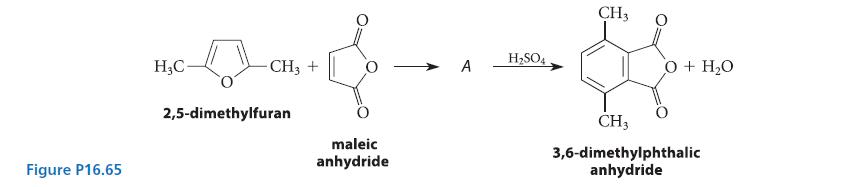
![(a) hexahelicene [a] =3700 degrees mL g- dm-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/6/7/0/302656d6d9ee8e731701670301706.jpg)