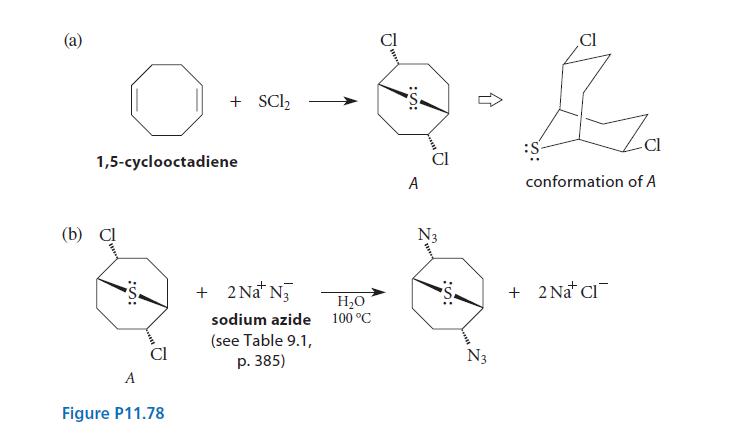
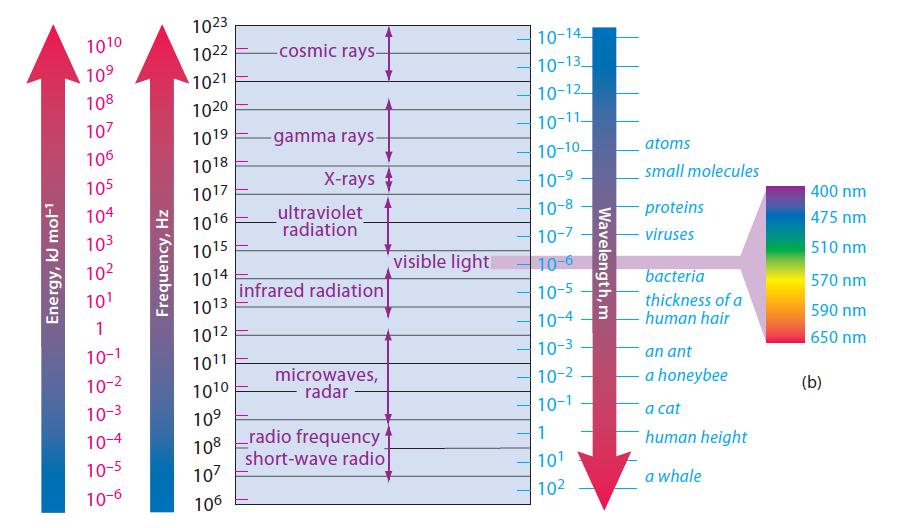
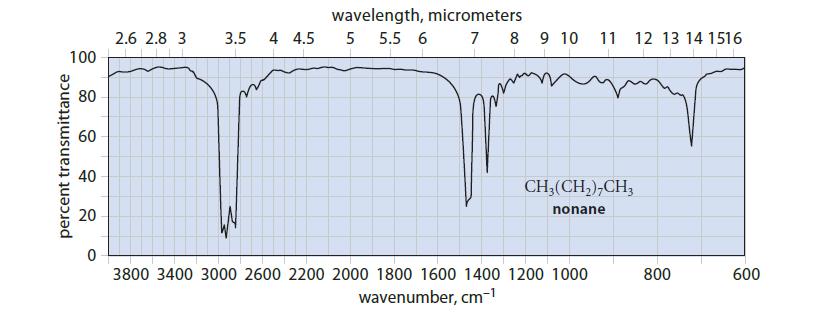
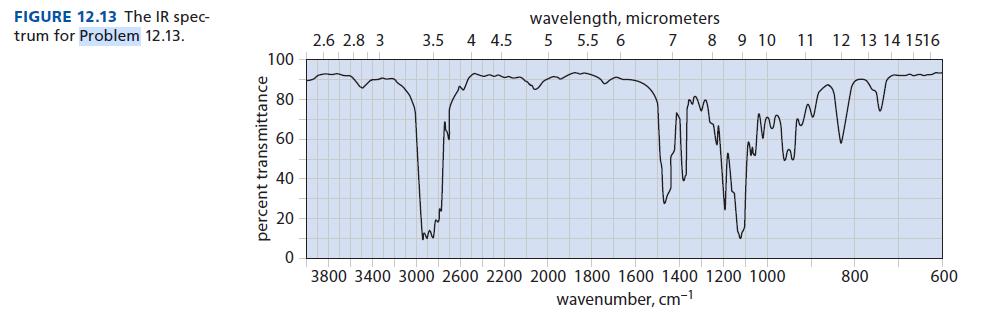
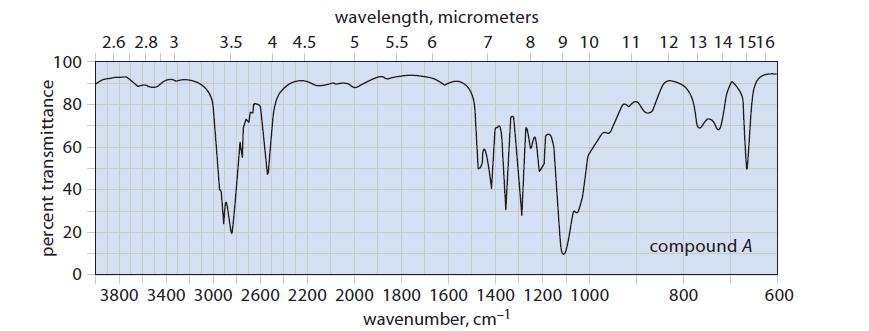
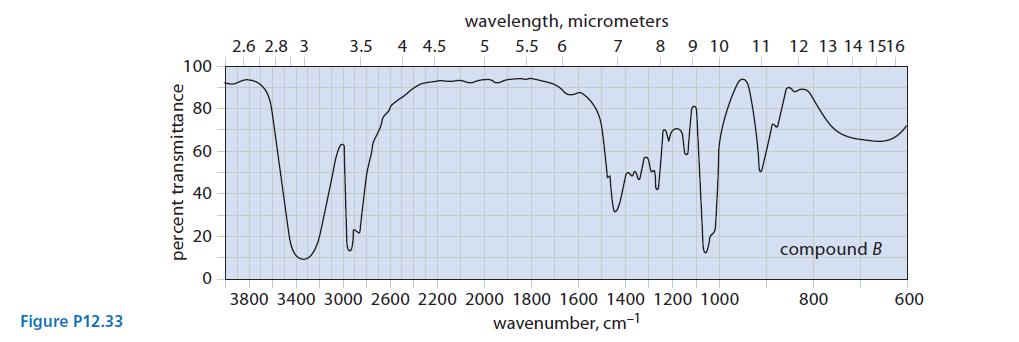
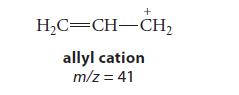![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


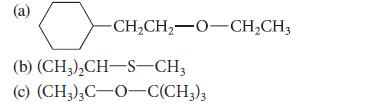
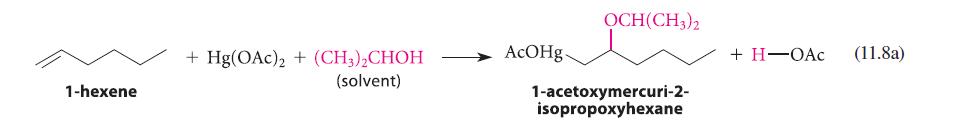
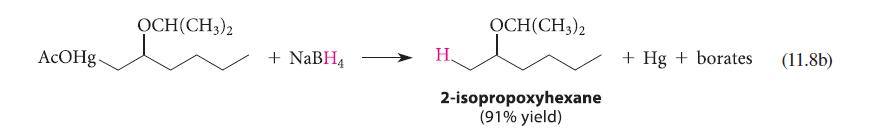
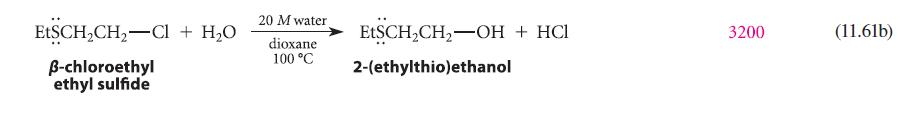
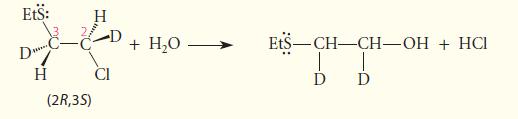
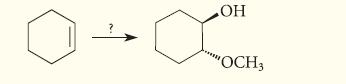
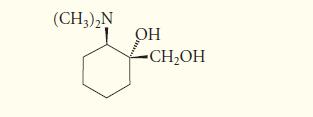
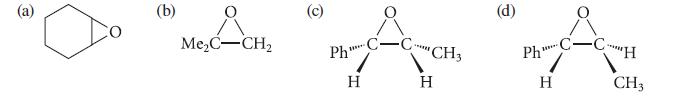
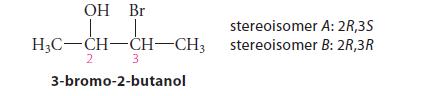
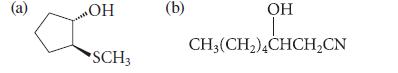
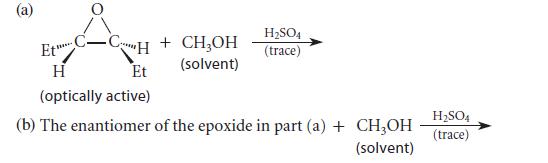
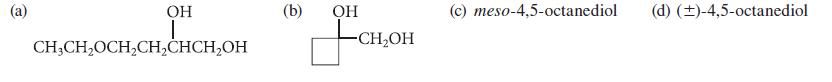
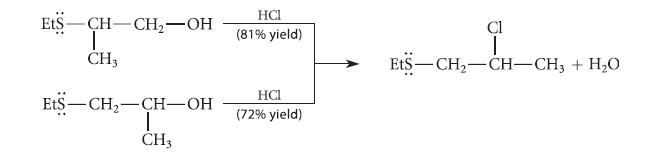

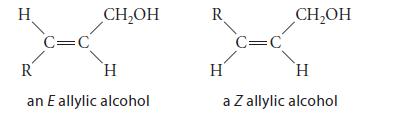
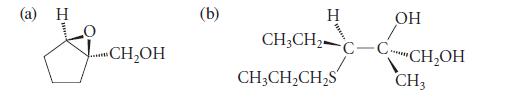
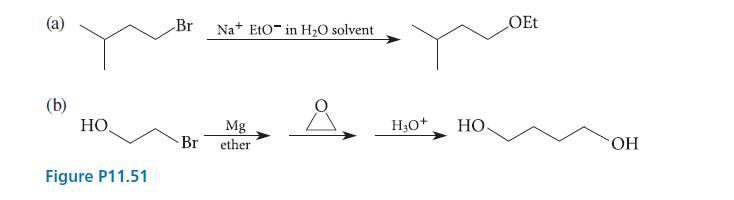
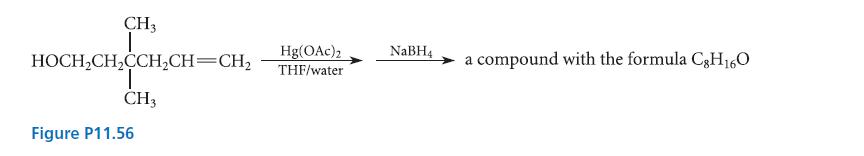
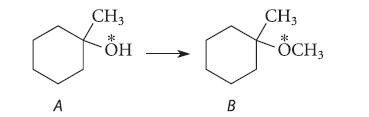
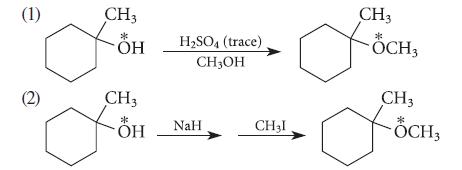
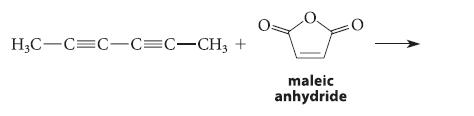
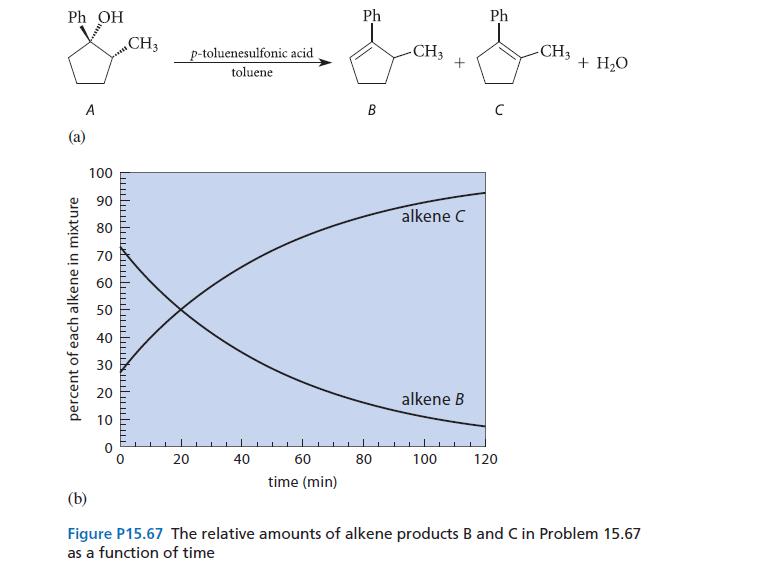
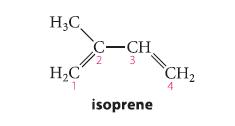
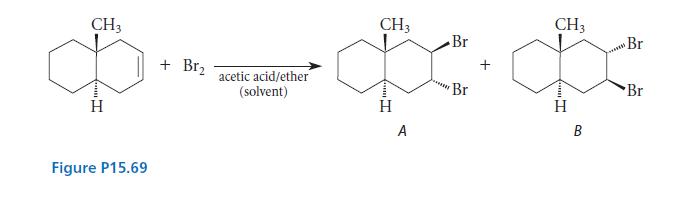
![* + K-H Figure P15.72 [X] + a gas HO * 20% +* 40% 40%](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/4/3/2/8526569ce14857d61701432851459.jpg)
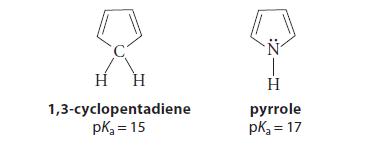
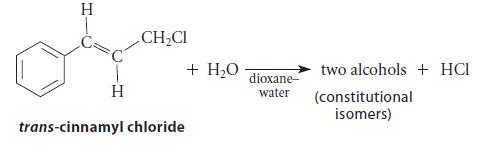
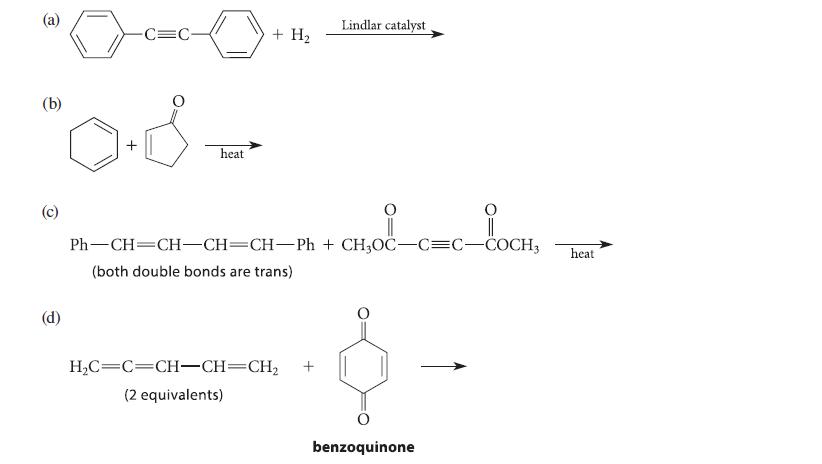
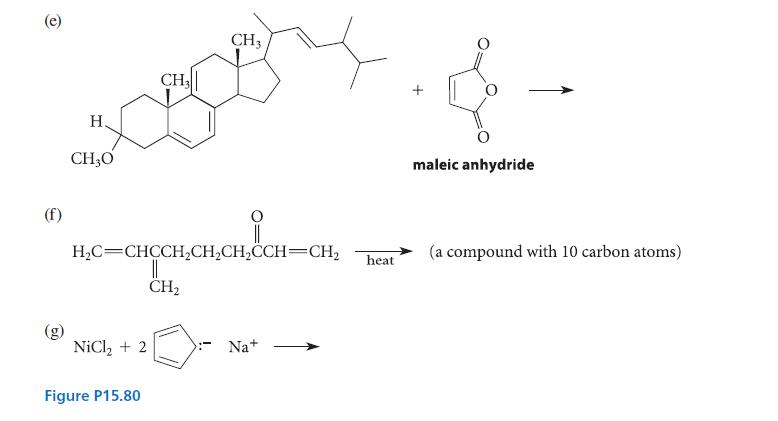
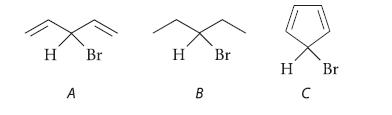
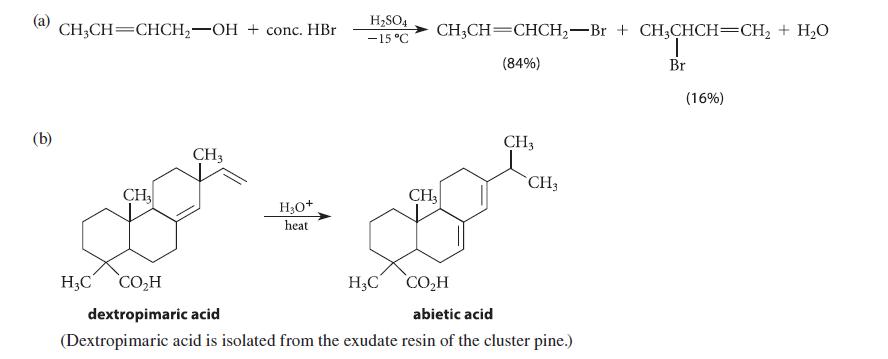
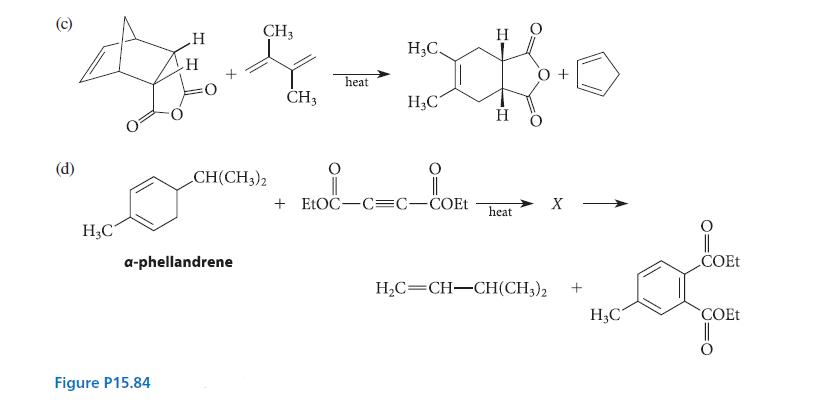
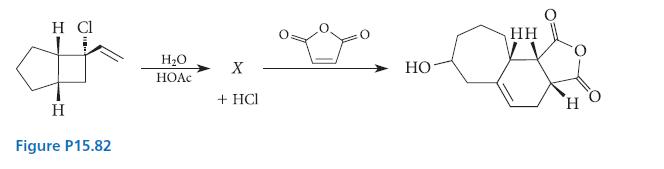
![[4]phenylene Figure P15.86 + 3H 5% Pd/C THF H H HH H H](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/4/3/3/3666569d016105371701433361598.jpg)