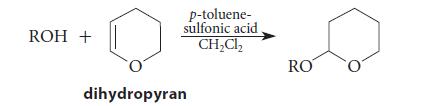
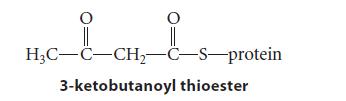
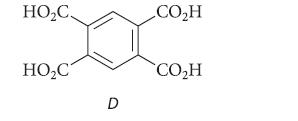
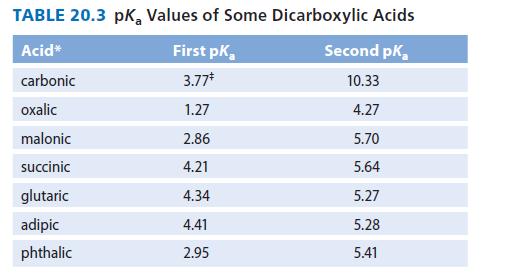
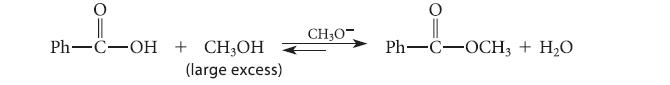
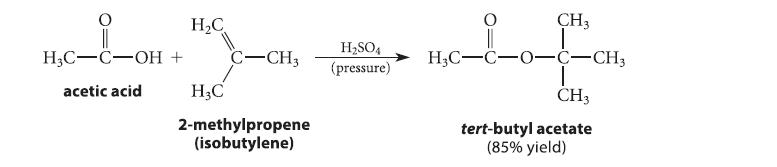
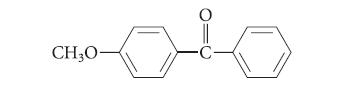
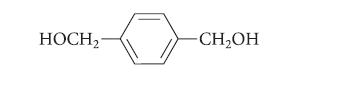
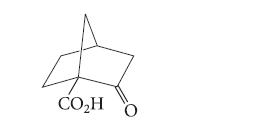
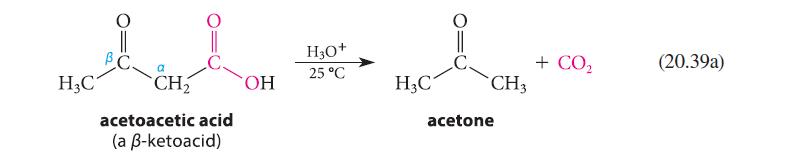
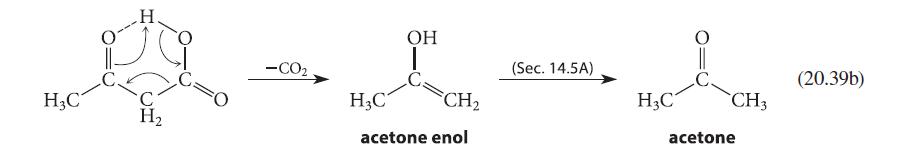
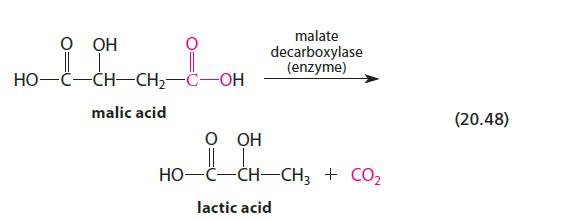
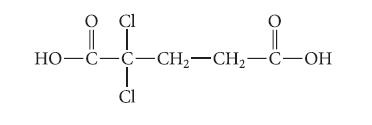
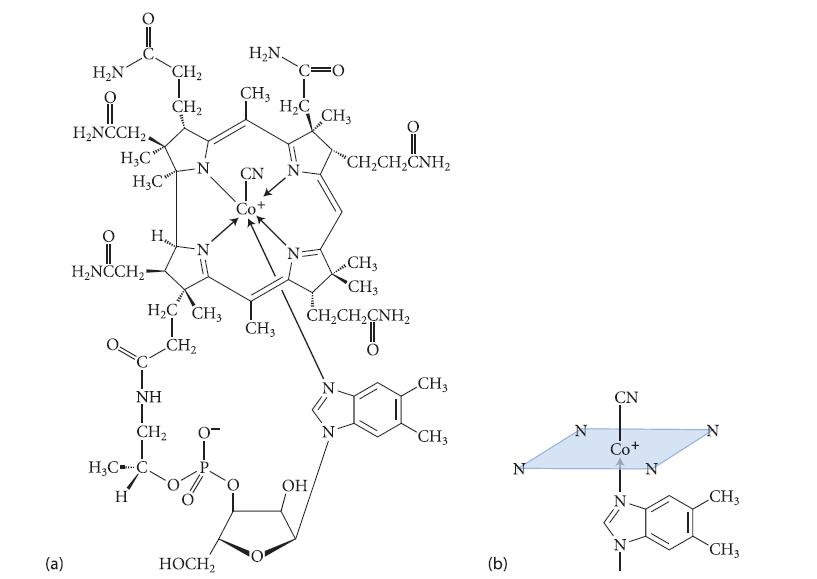
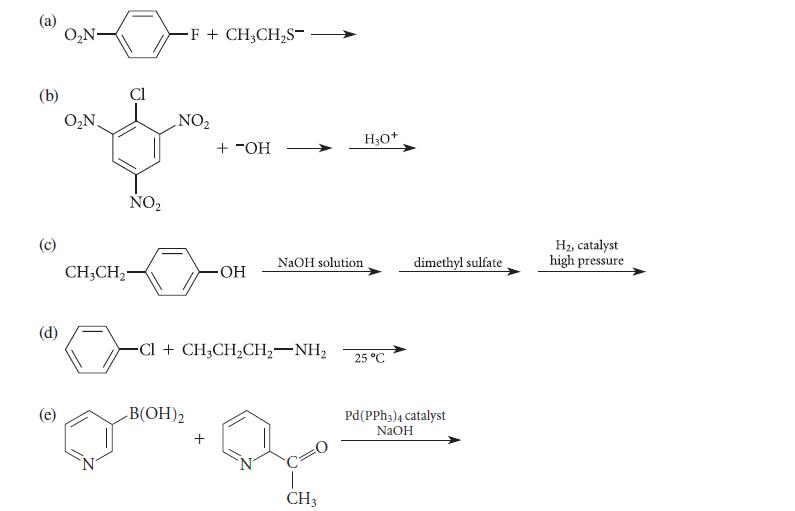
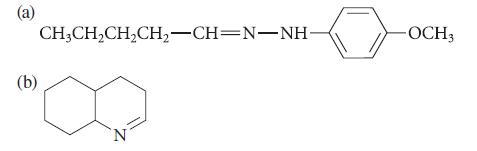![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


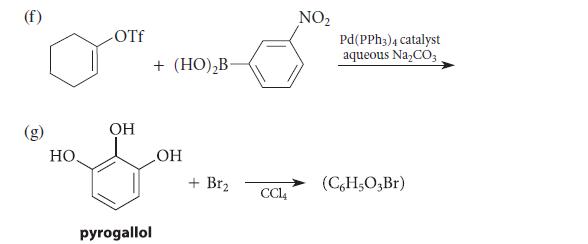
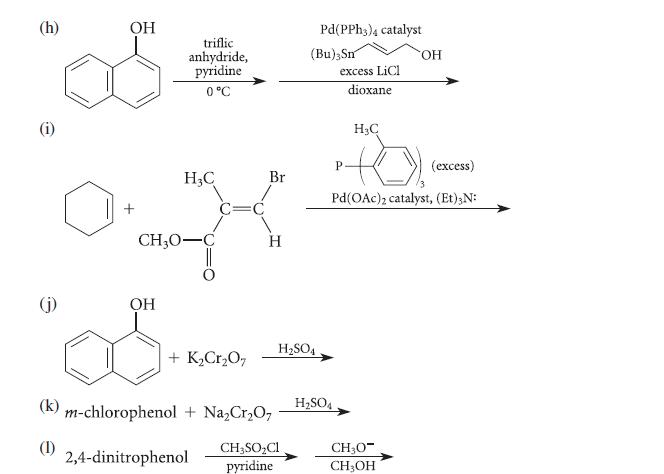
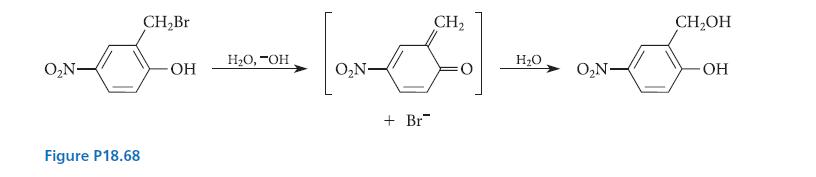
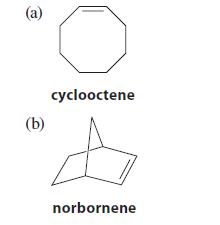
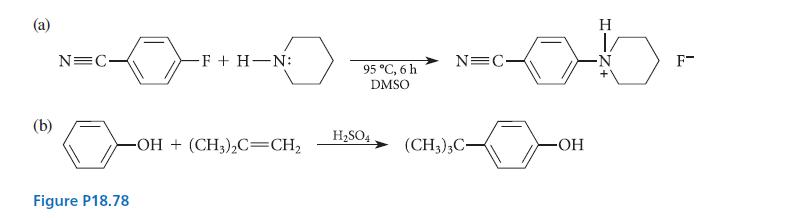
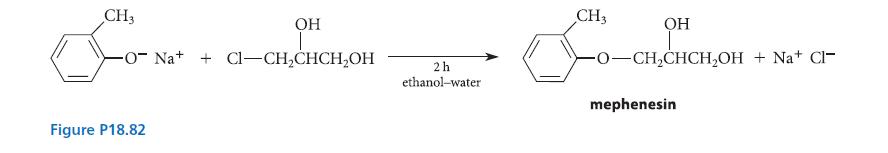
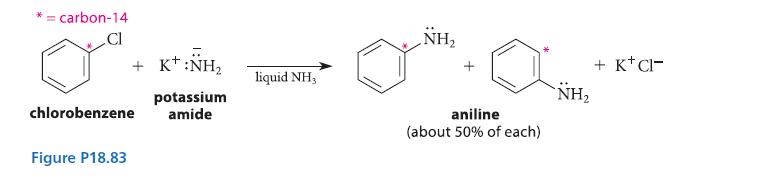
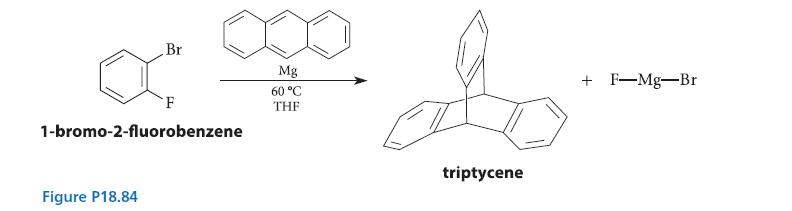
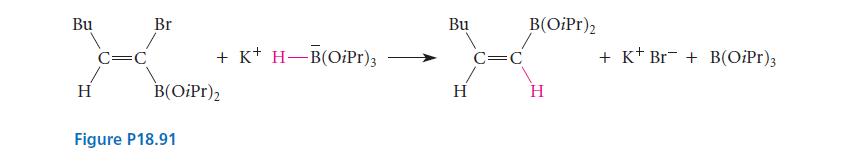
![X + C=C H C=C-H+ [iBu]Al-H Figure P18.76 H diisobutylaluminum hydride (DIBAL) Pd(PPH3)4 catalyst X Y](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/6/8/6/190656dabae943221701686188292.jpg)
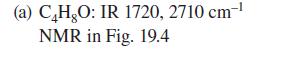
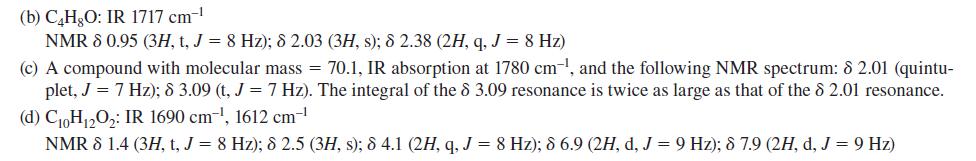
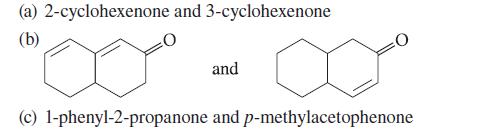
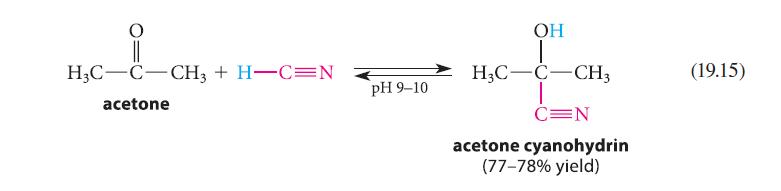
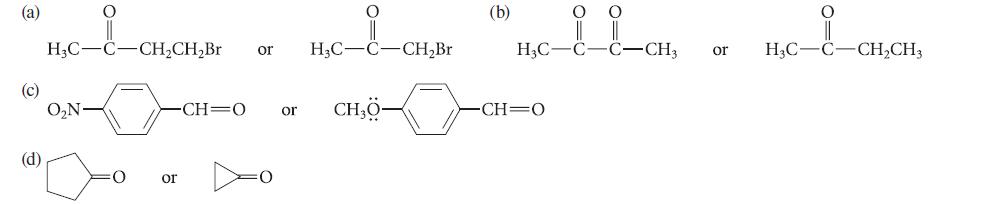
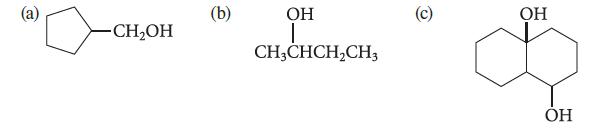
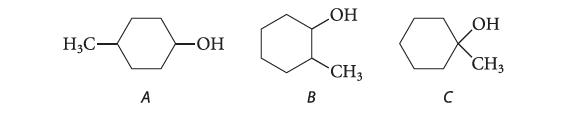
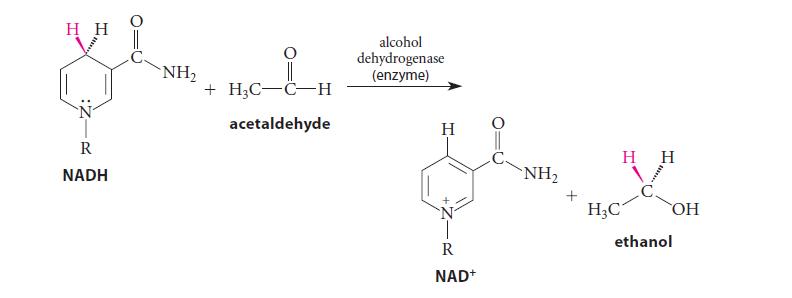
![:0: Na H-S-0:- :O: Figure P19.42 :0: Na S-OH + R-CH Im]. :0: :0: || sodium bisulfite :H | R-CH o=s=0 :0:- Na+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/7/5/5/273656eb98931b251701755272380.jpg)