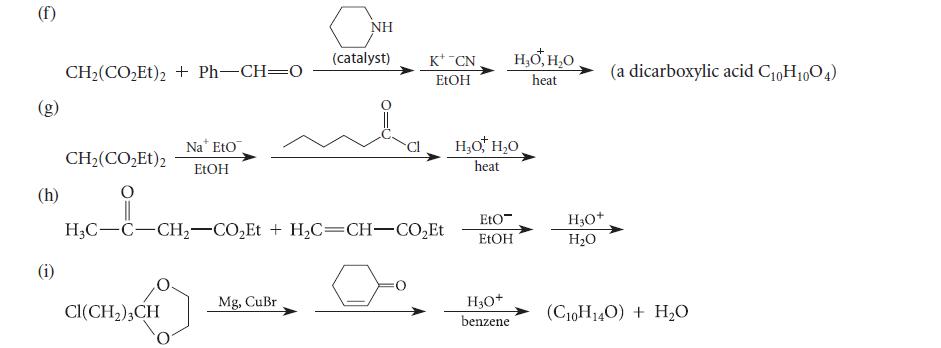
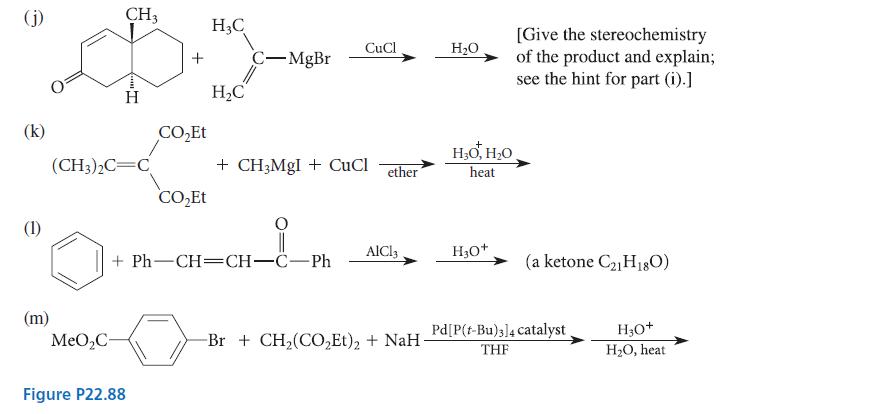
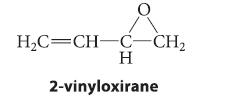
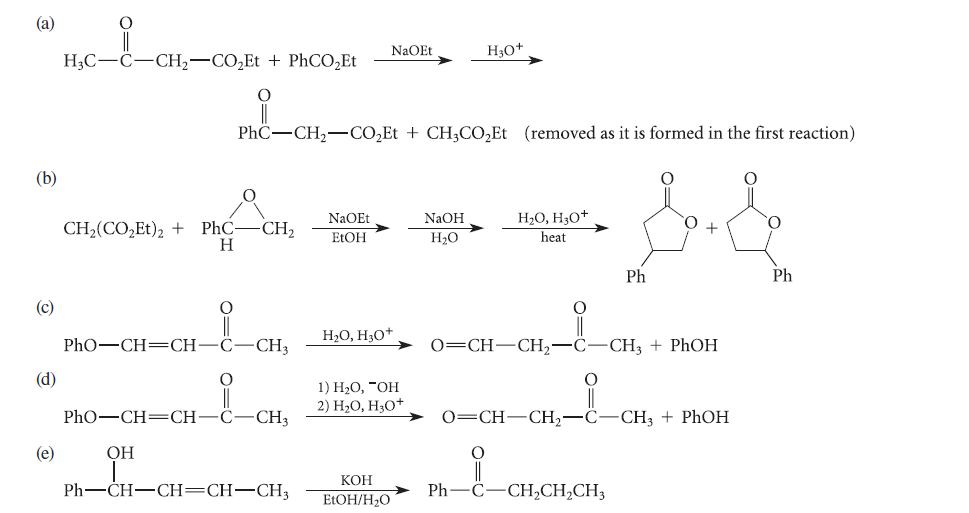
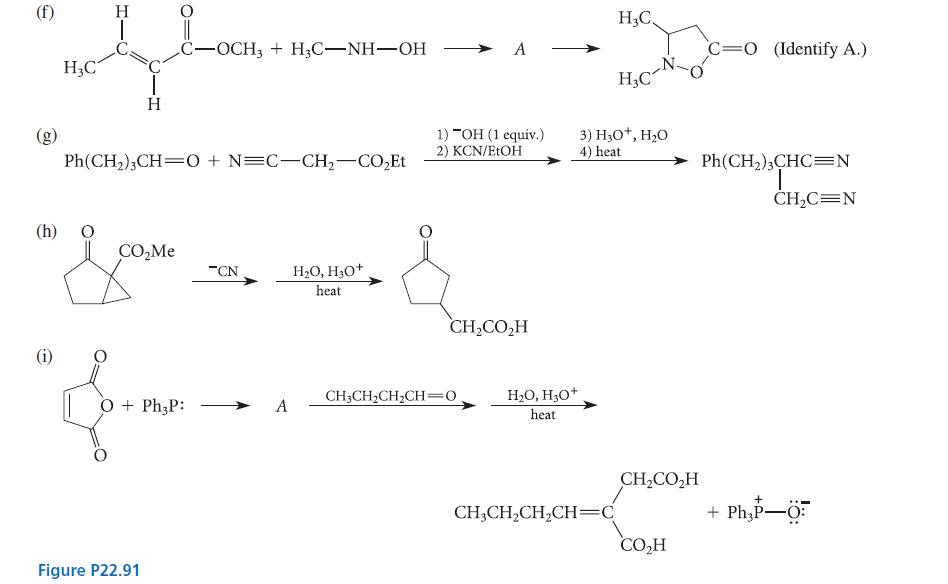
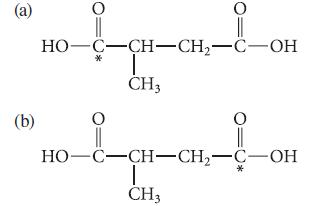
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
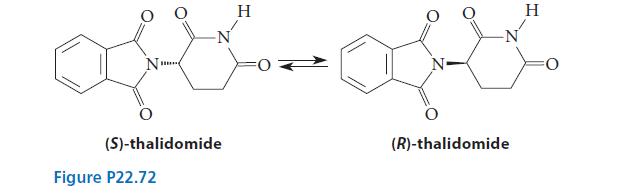
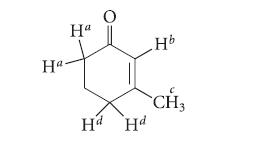
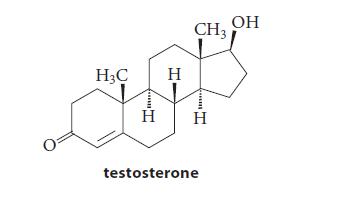
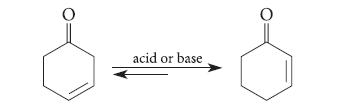
![]()


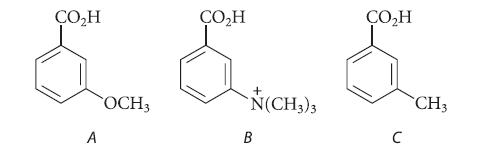
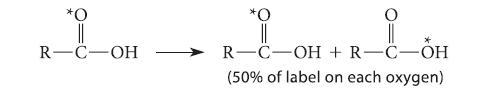
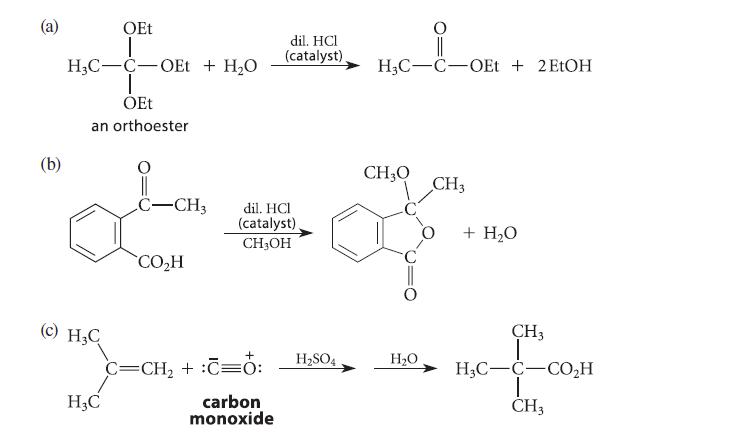
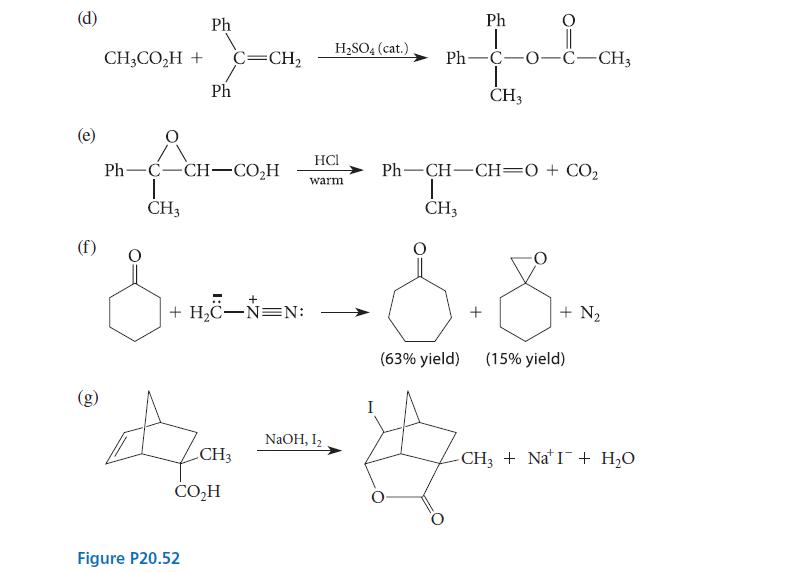
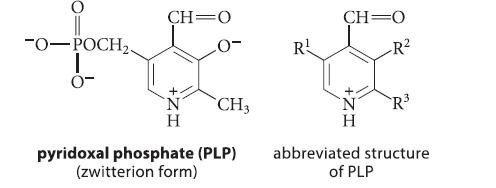
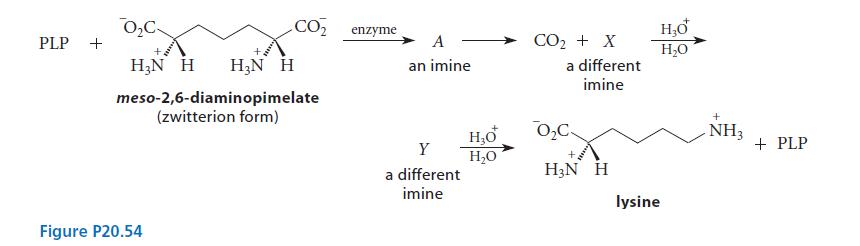
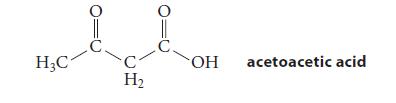
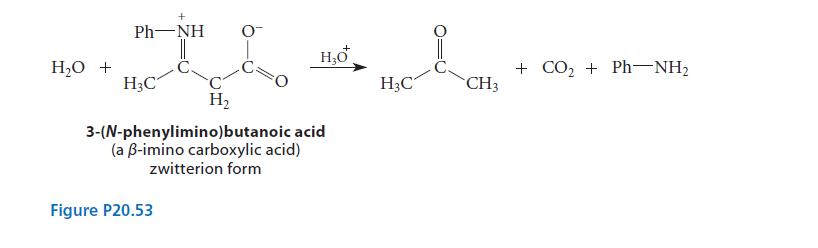
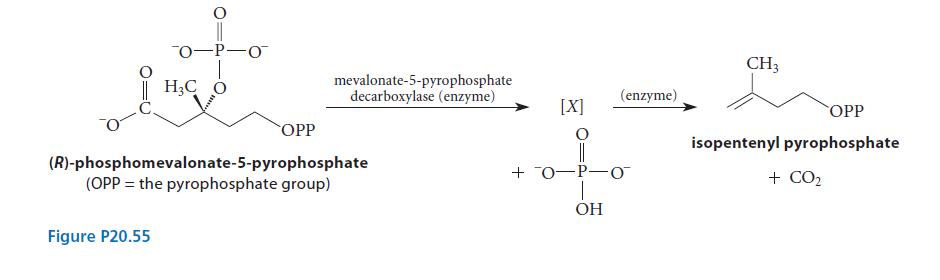
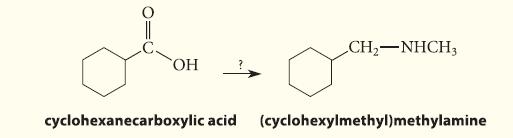
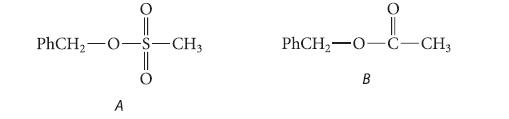
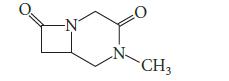
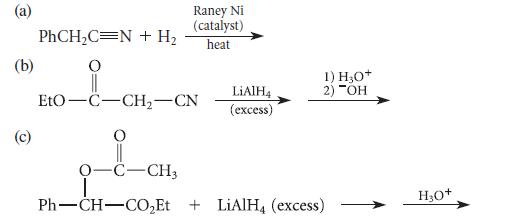
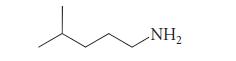

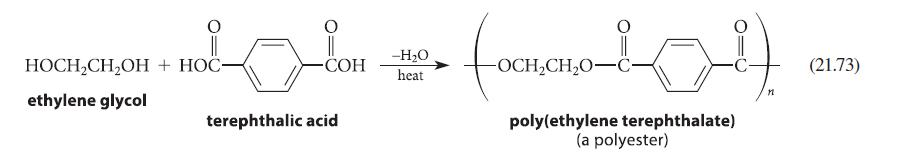
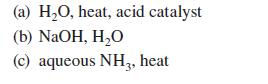
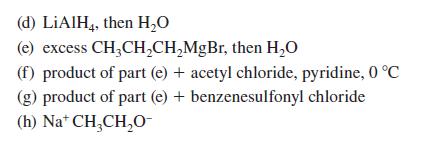
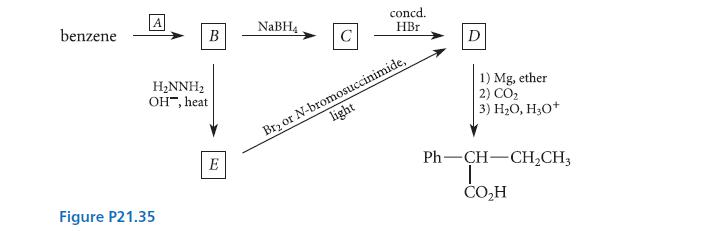
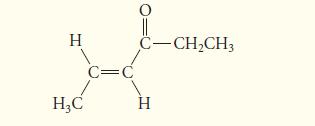
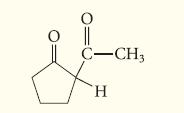
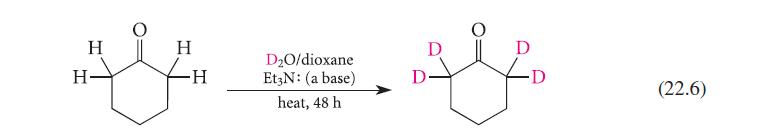
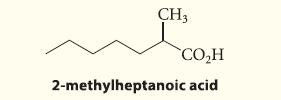
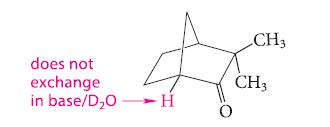
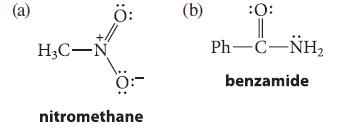
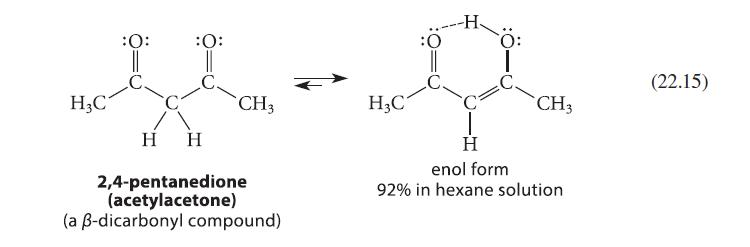
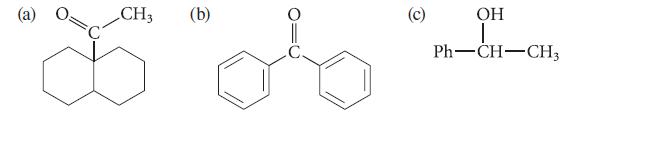
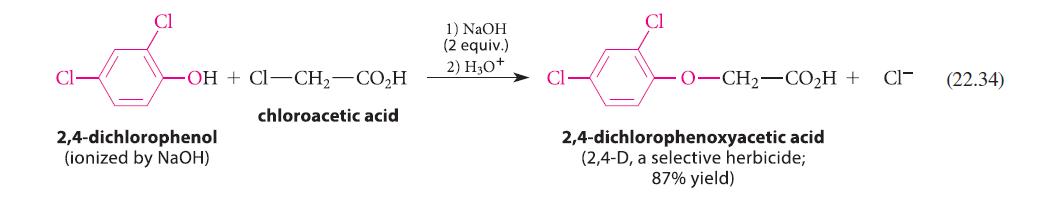
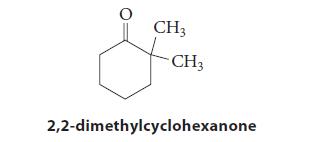
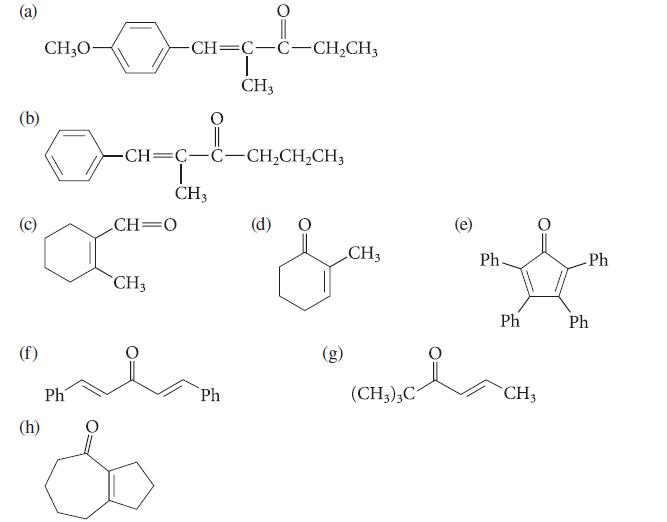
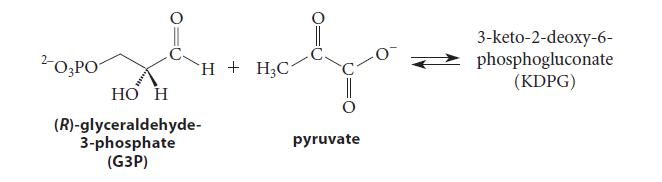
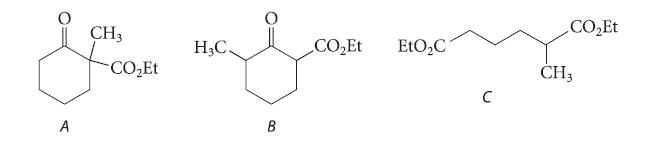
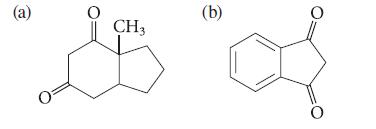
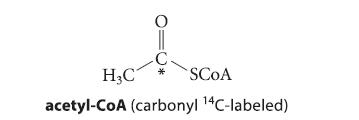
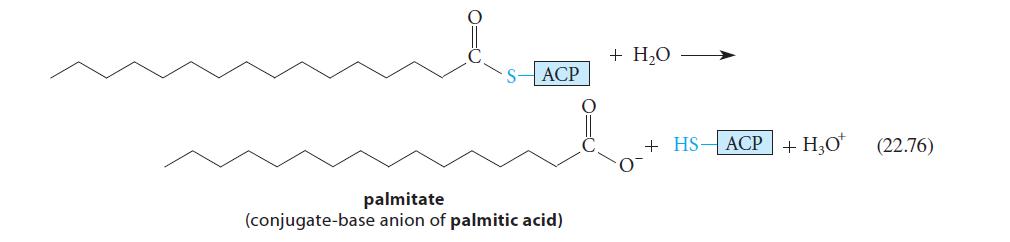
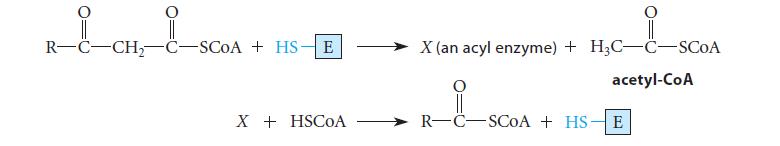
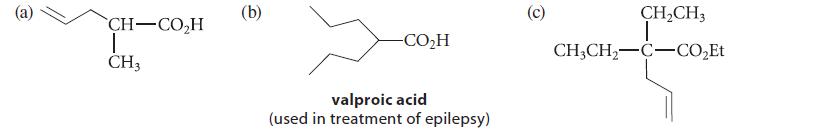
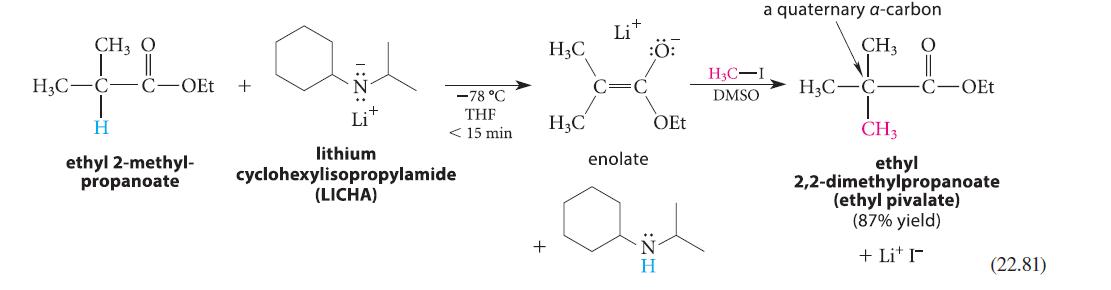
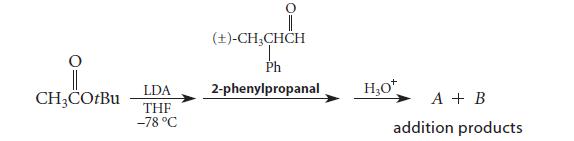
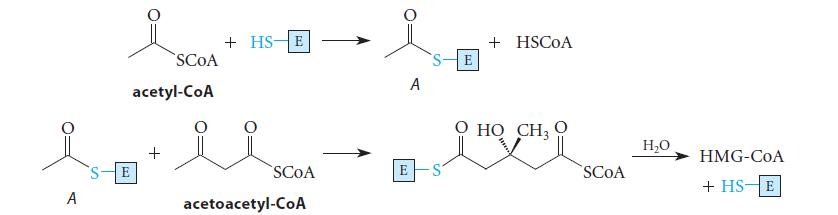
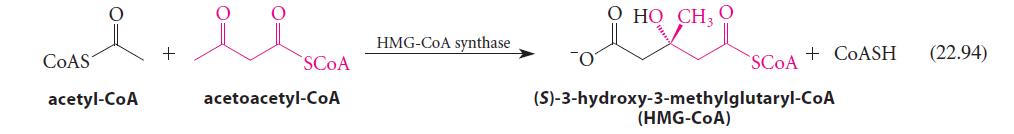
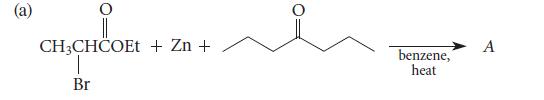
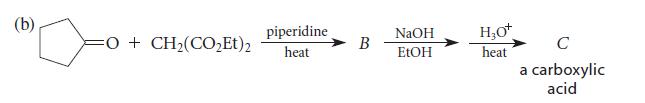
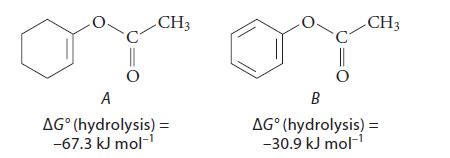
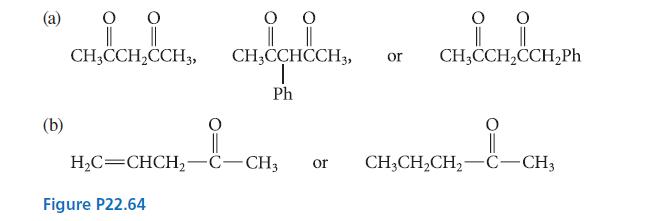
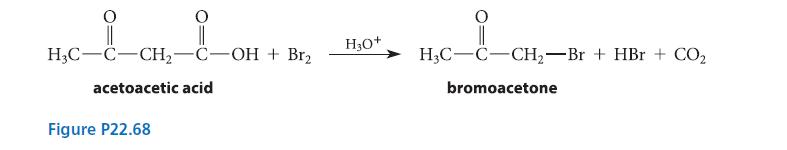
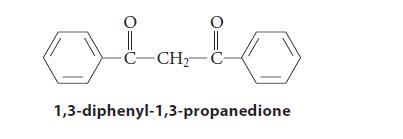
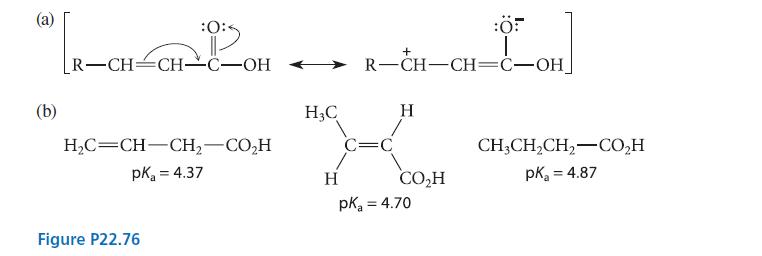
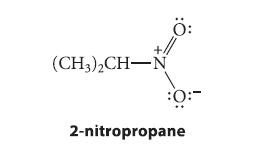
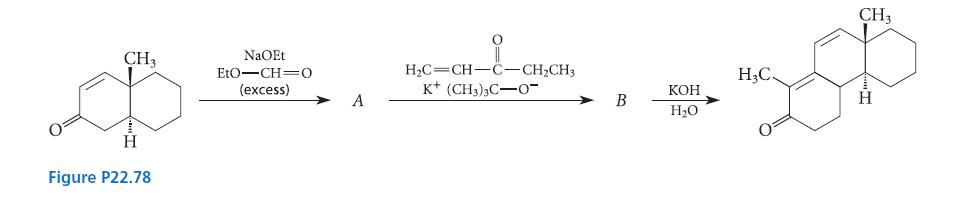
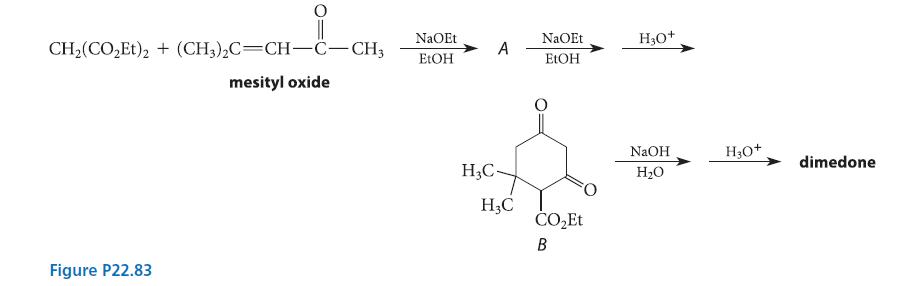
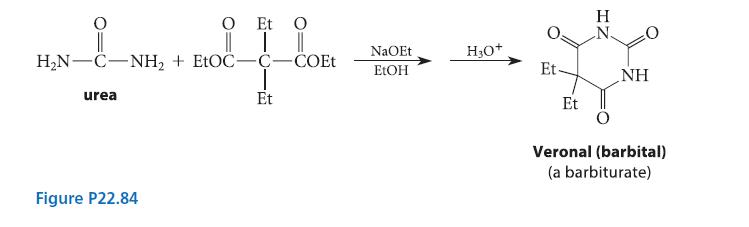
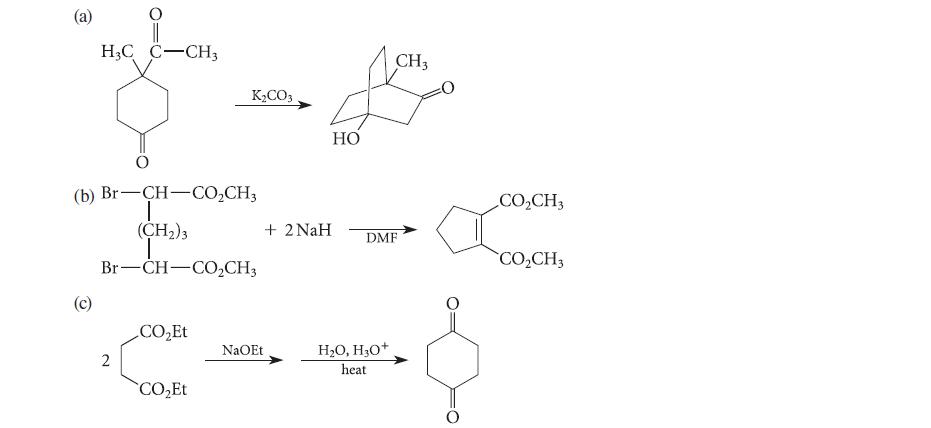
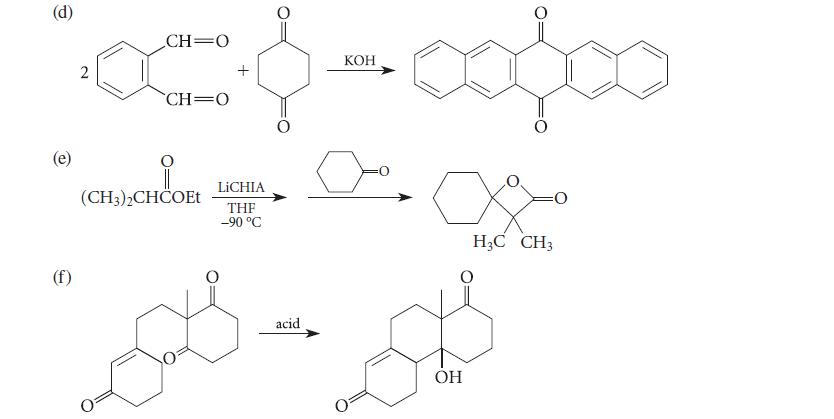
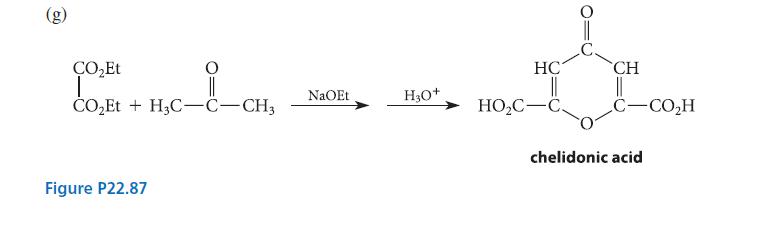
![(b) (c) (d) (e) 1-CH-i H3C-C-CH-C-OEt + Br(CH)4Br & y-butyrolactone Cl NO CH3 NO Li* [(CH3)zCH],N: +Na+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/6/4/5986570649613da71701864595336.jpg)