![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


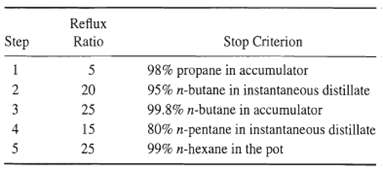
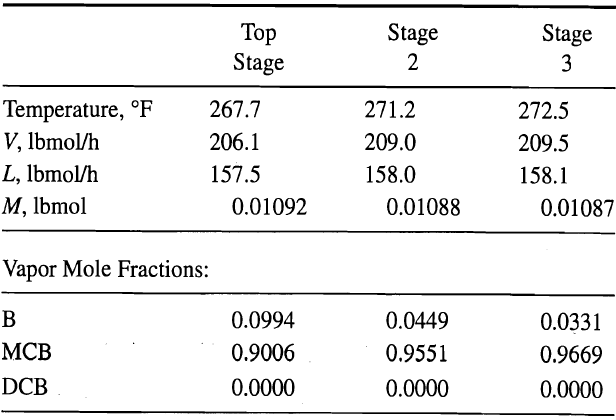
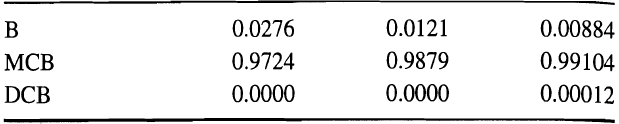

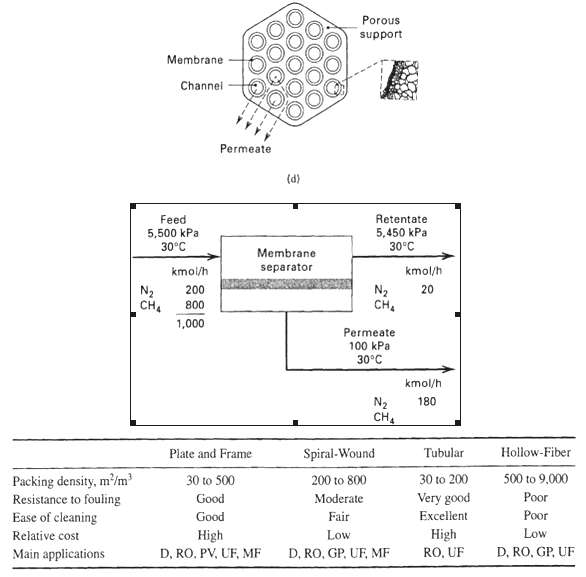
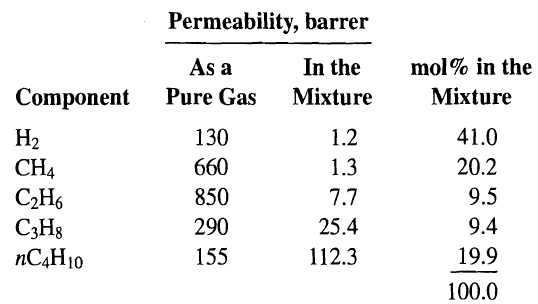
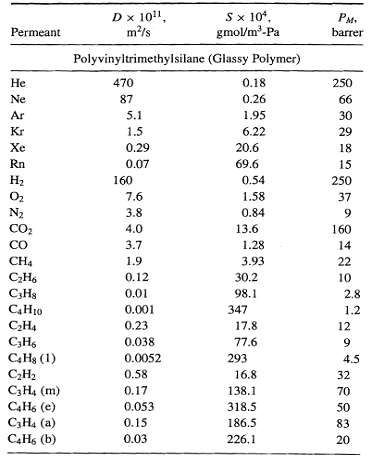
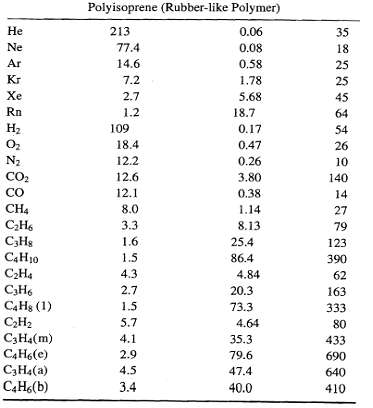
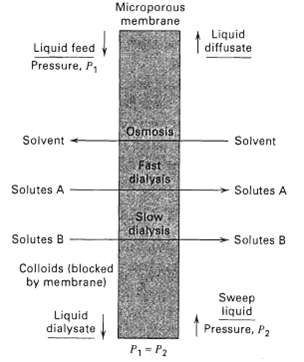
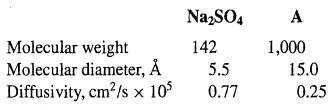

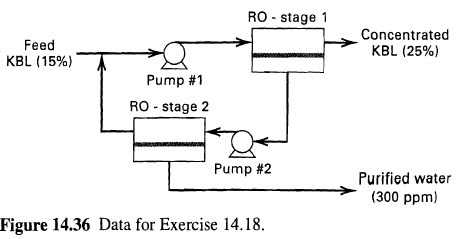

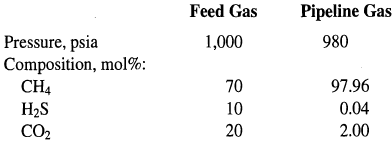


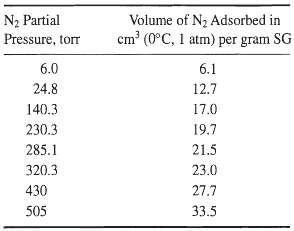
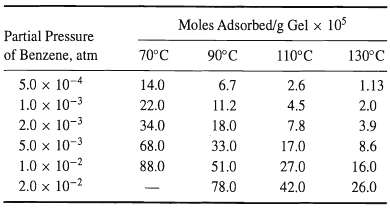
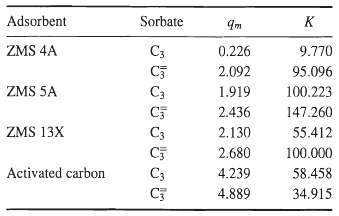
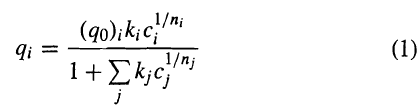
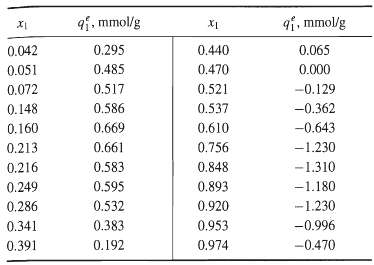
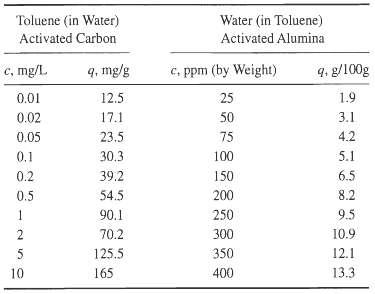
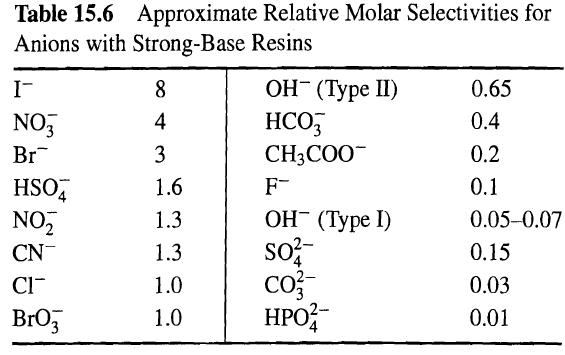

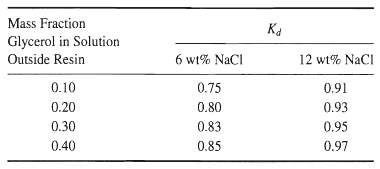
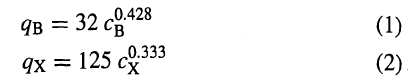
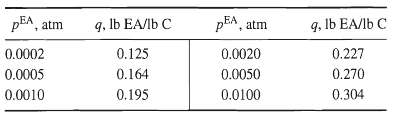
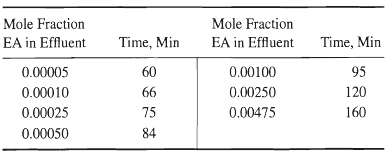
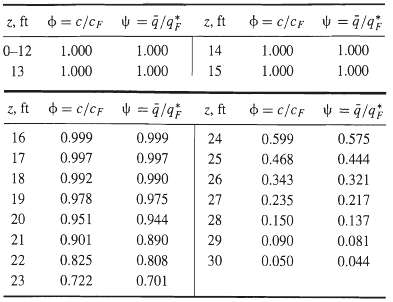

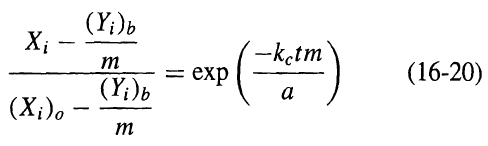
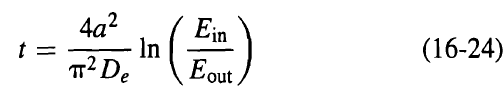
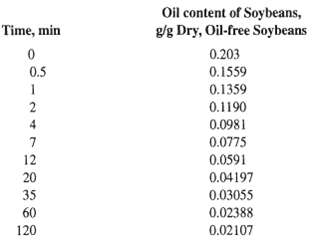
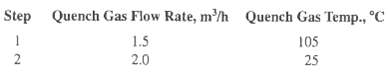
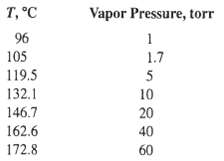
![[4)-6-a] .2 (:) p.ΔΗ (17-75) In ro ke(Tg – Te) [ 2 [e-A}-](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1554/2/0/8/4885ca356e8657bd1554208488349.jpg)