![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


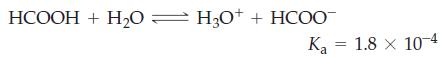
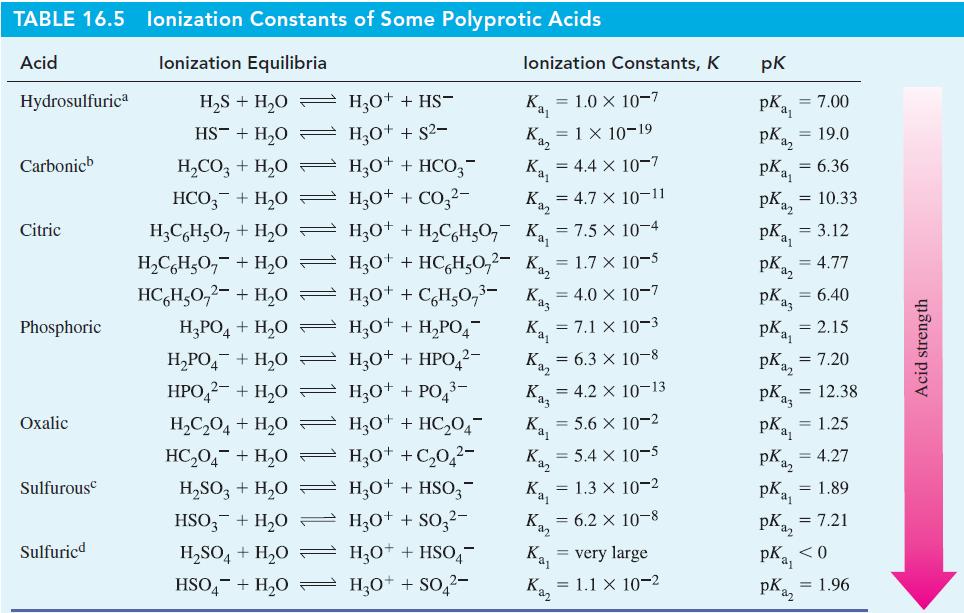

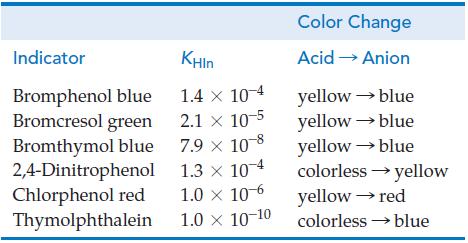
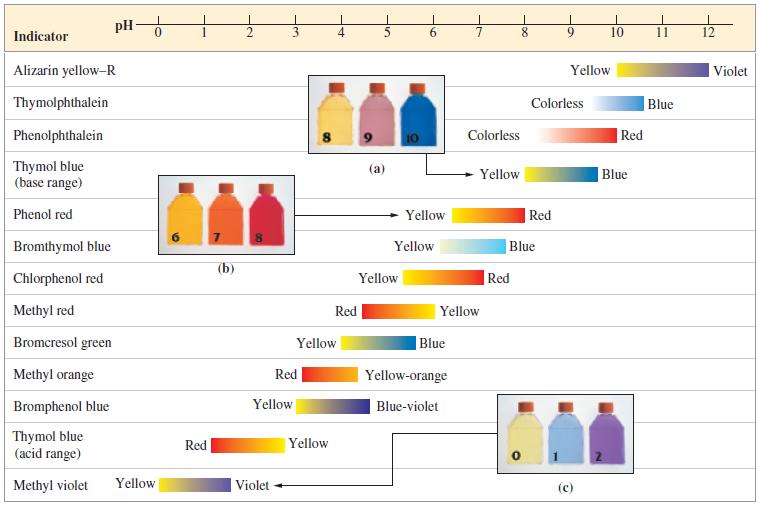
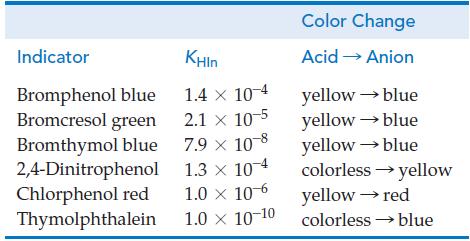
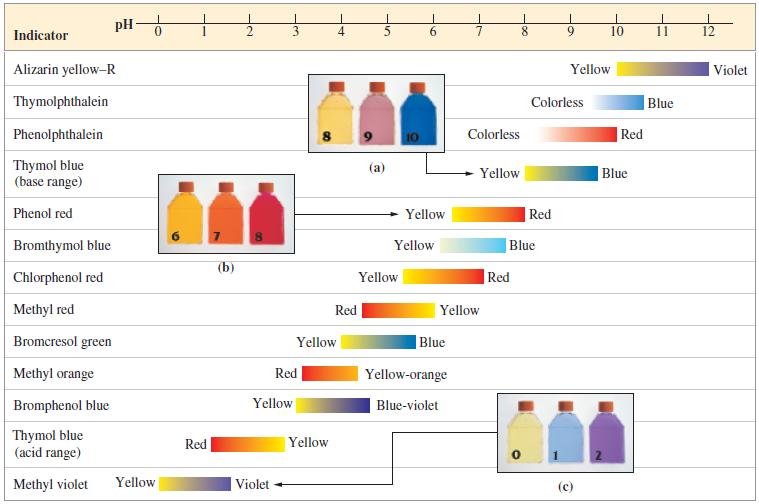
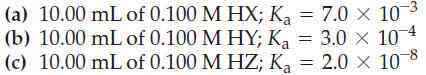
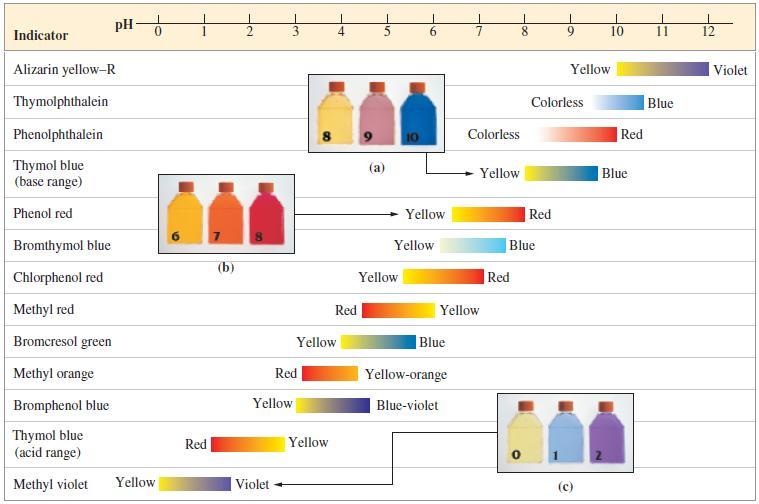

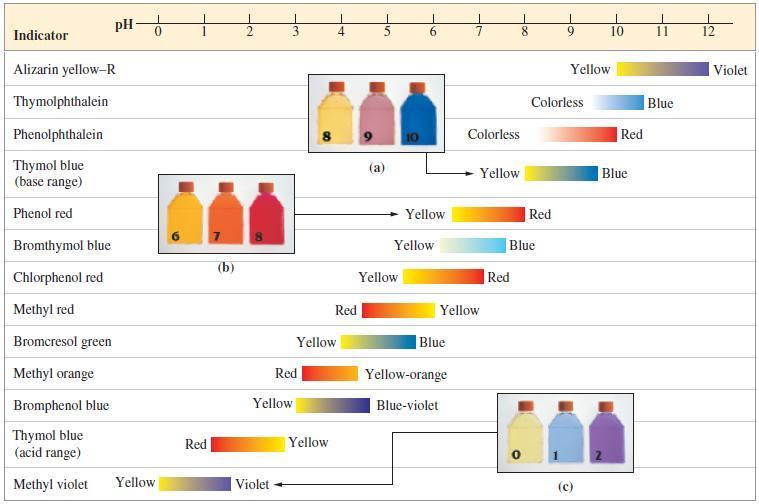
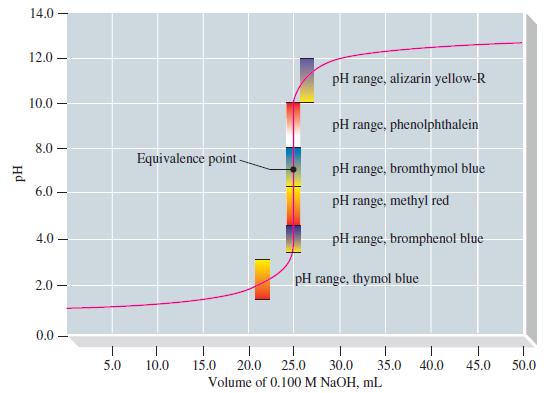
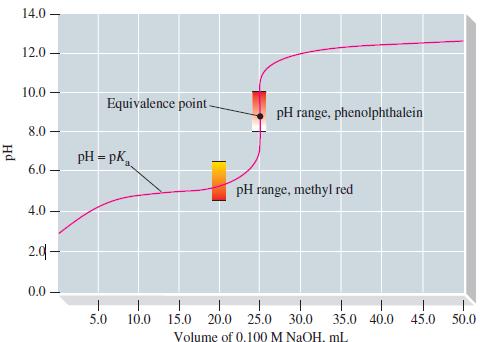
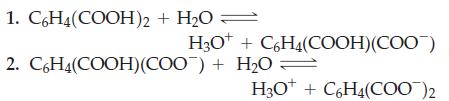

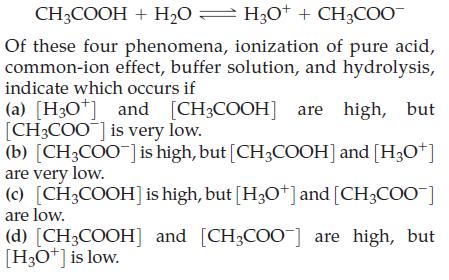
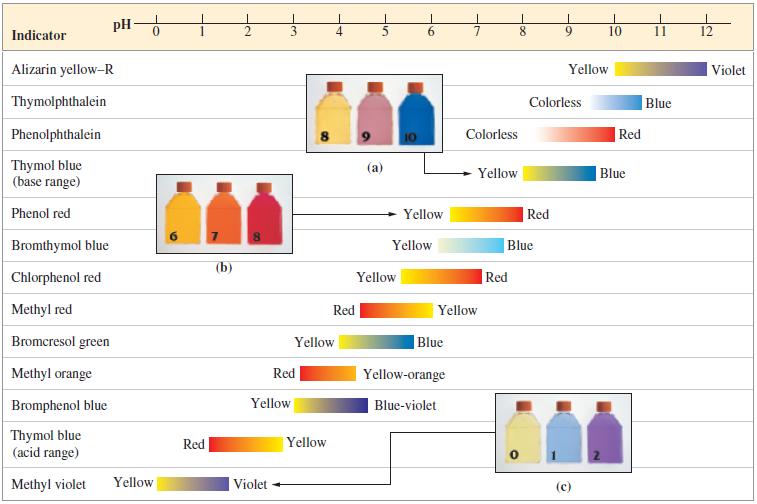
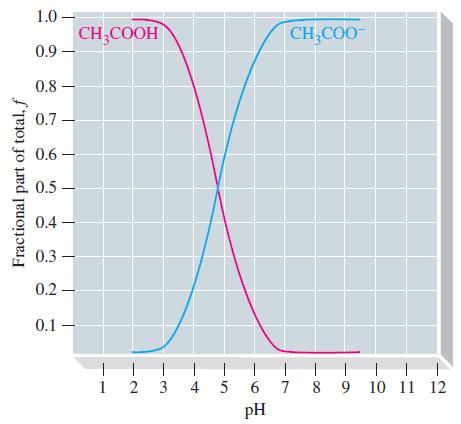
![[conjugate base] [acid] pH = pka + log- (17.7)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/6/4/9/296655dd950351981700649294733.jpg)
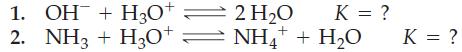
![HN(CH8) NH + HO = [HN(C4H8) NH]+ + OH [HN(C4H8) NH] + HO = [HN(C4H8) NH]+ + OH pKb pKb = 8.67 = 4.22](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/6/5/0/181655ddcc5eaf861700650180966.jpg)
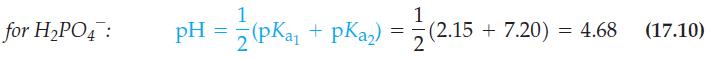
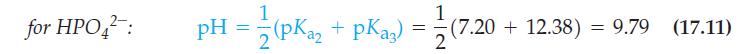
![08(21/1 -1) pH = pKa - log where a [A] [A] + [HA]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/6/5/1/663655de28fad5801700651662613.jpg)
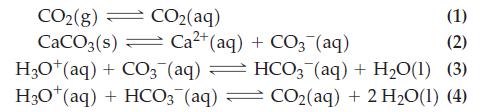
![B = 2.303 Kw [H3O+] + [H3O+] + cKa[H3O+] (Ka+ [H3O+])2/](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/6/5/3/202655de89261ff71700653201342.jpg)