![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


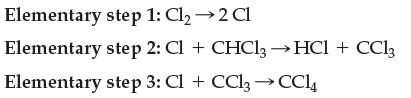
![3H(8)+N(8) Keq 2 NH3(8) [NH3) [H][N] = 0.00016 (at 400C)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/4/0/52465718d2cec50a1701940526224.jpg)
![(a) K = [P][0]/[P4010] (b) K = 1/[0] (c) K = [0]5 (d) K = [P]/[P4O10]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/4/0/09065718b7a4c5531701940090532.jpg)

![Rate forward rxn kforward rxn [Reactants]order and Rate reverse rxn = Kreverse rxn[Products Jorder](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/7/0/22965707a9515c7e1701870227603.jpg)
![Kec req _[HO1x [C11 [HC] x[0]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/2/6/483657156535f4d71701926482927.jpg)
