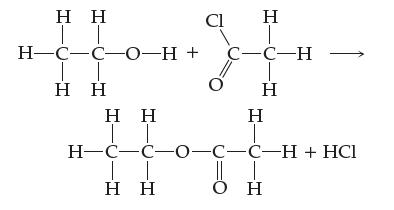
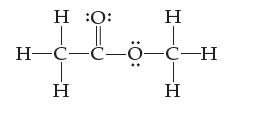
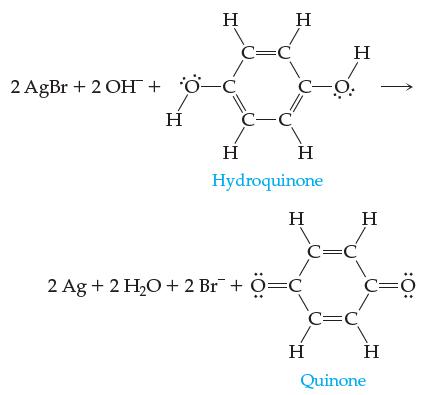
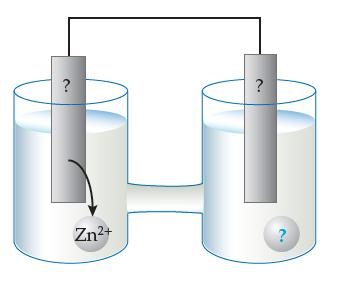
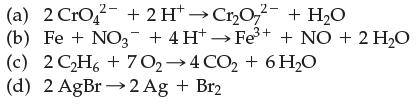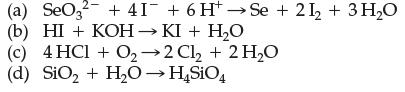![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![Initial Experiment [CHBr] 1 0.200 M 2 0.400 M 3 0.400 M Initial [OH-] 0.200 M 0.200 M 0.400 M Rate of CH3OH](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/6/8/224657072c08f6631701868223119.jpg)
![Experiment 1 2 3 4 Initial [NO] 0.015 M 0.030 M 0.015 M 0.030 M Initial [0] Rate of NO formation (M/s) 0.015](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/5/2/4786570353ee570b1701852476166.jpg)
![(a) CHI+ Cl CH3Cl + I (b) NH3 + CHI[(CH3)NH]*I](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/8/5/3/4706570391e8df931701853467001.jpg)