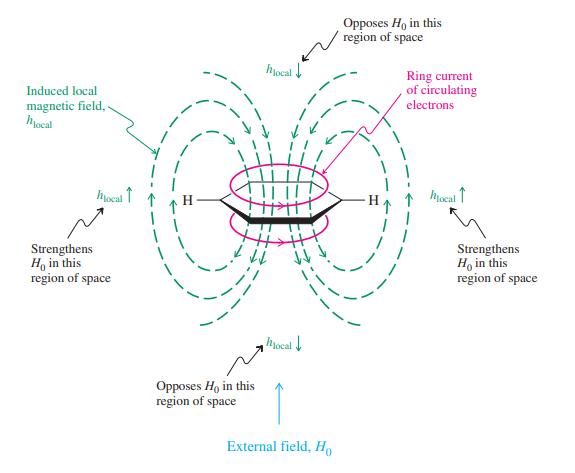
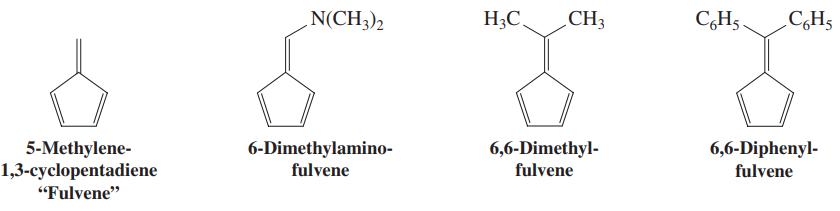
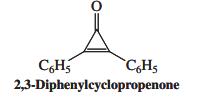
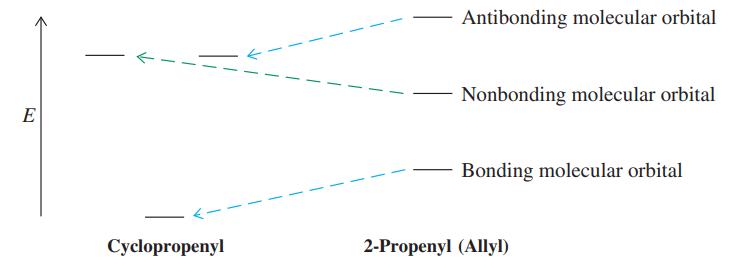
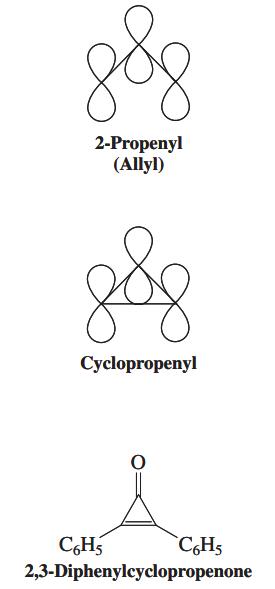
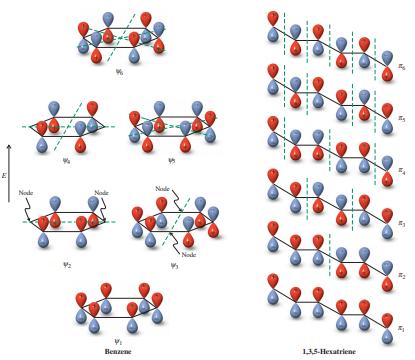
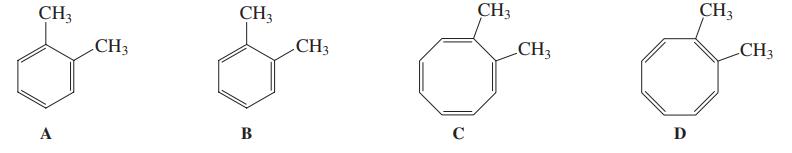
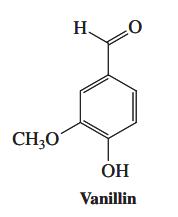
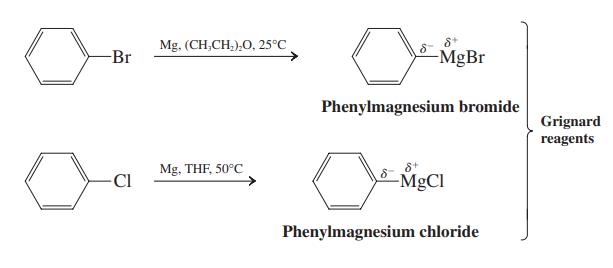
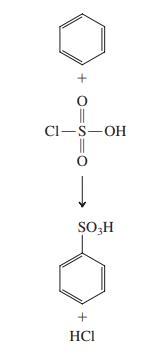
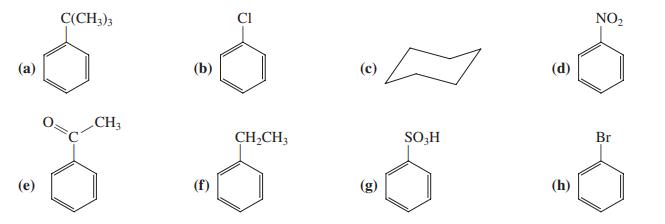
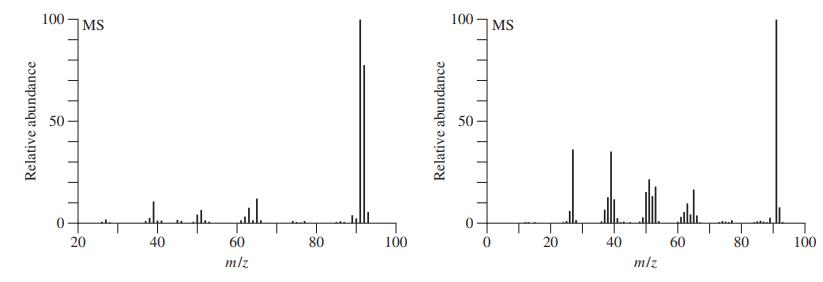
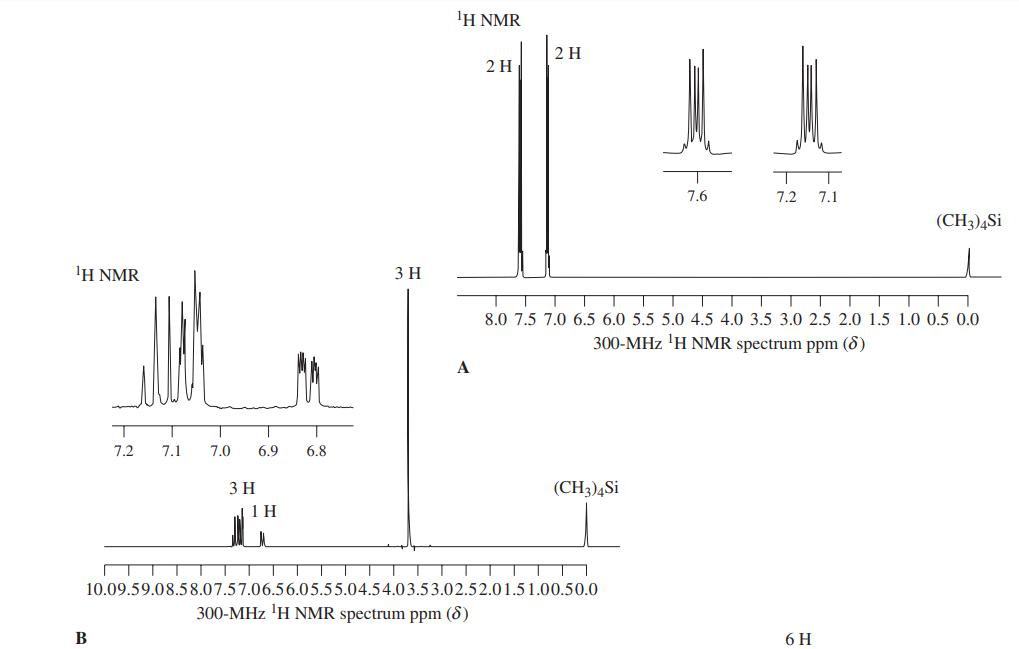
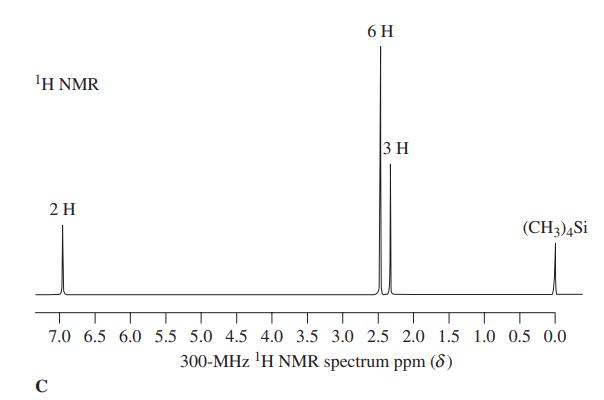
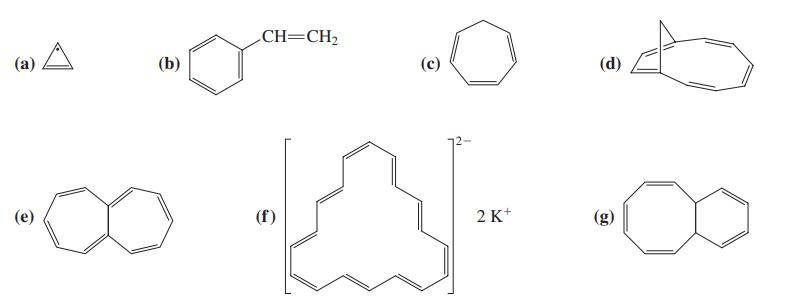
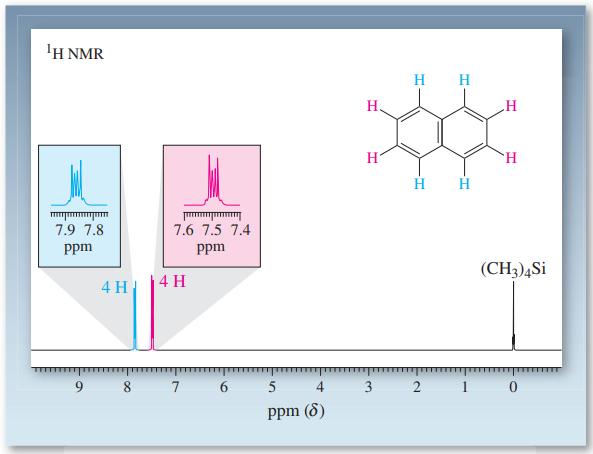
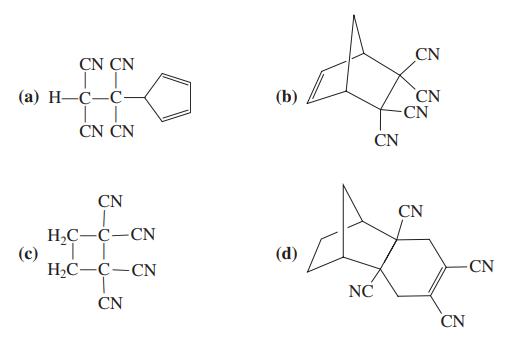
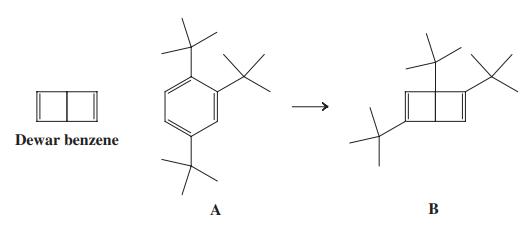
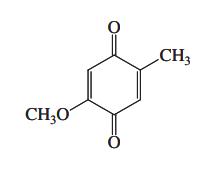
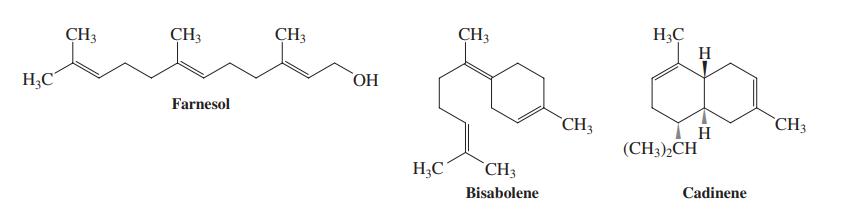
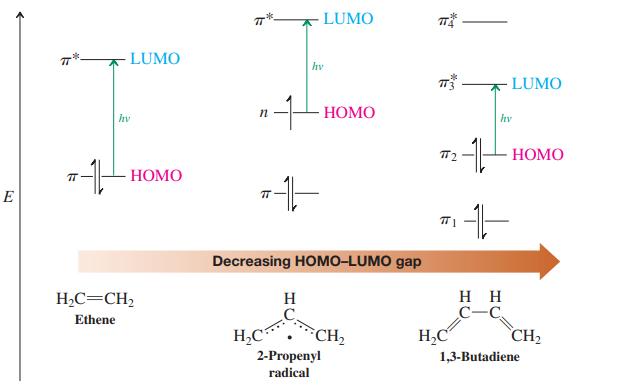
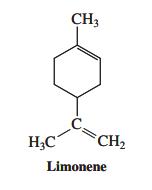
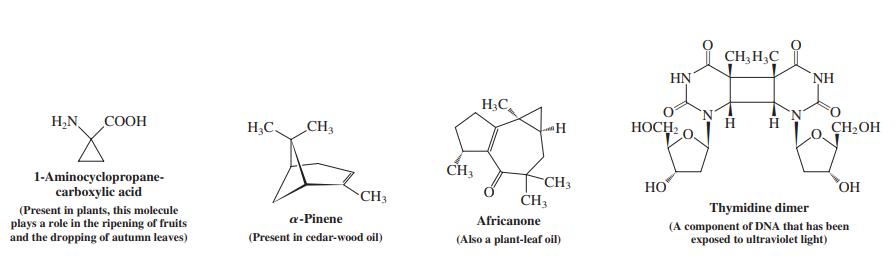
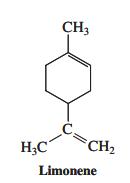
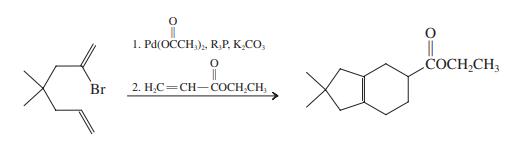
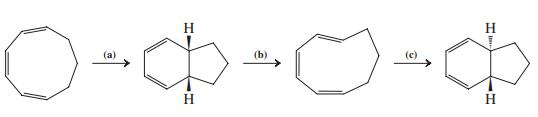
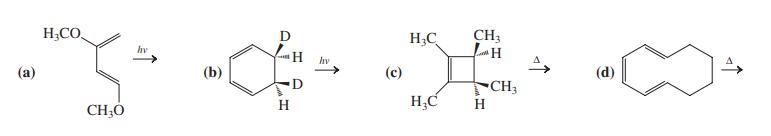
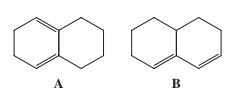
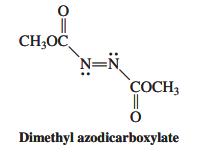
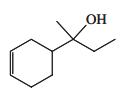
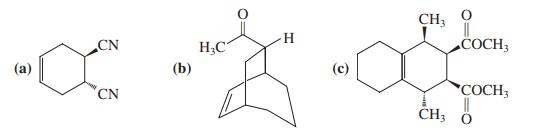
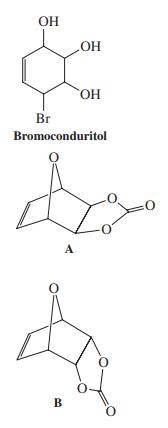
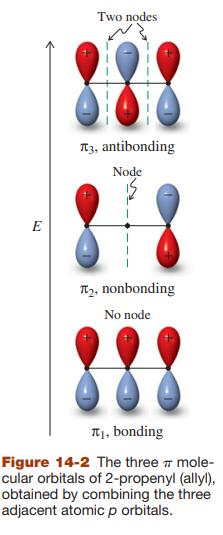
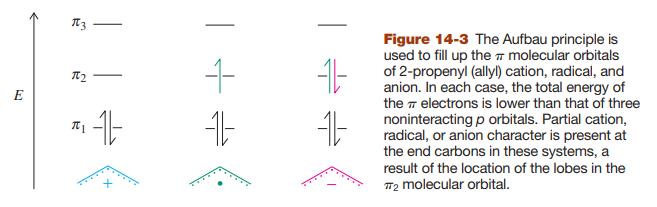
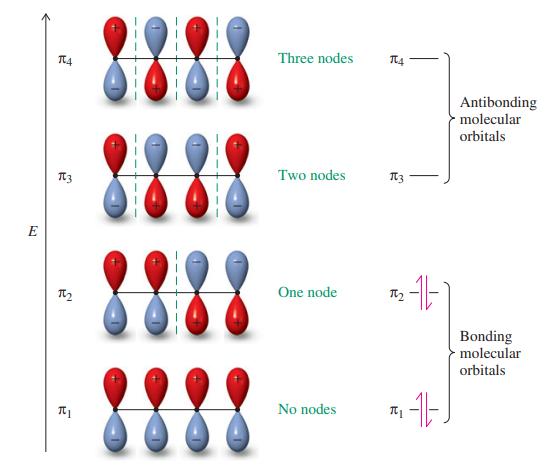
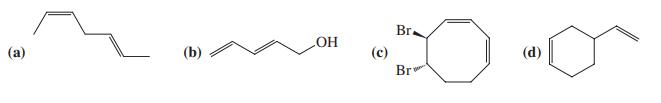
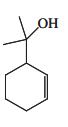
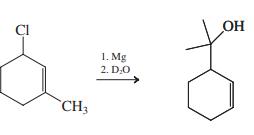
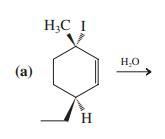
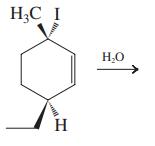
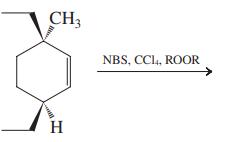
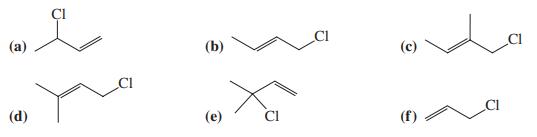
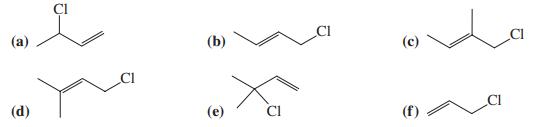
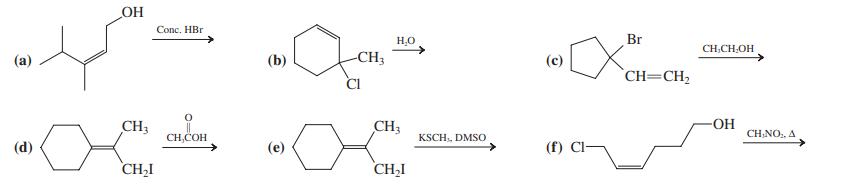
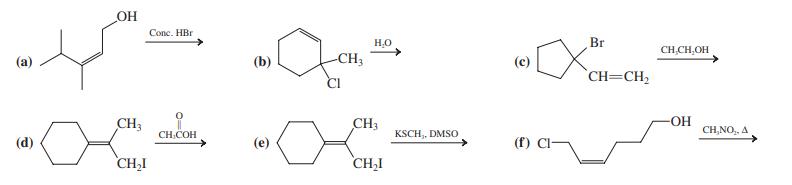
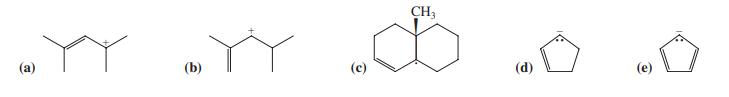
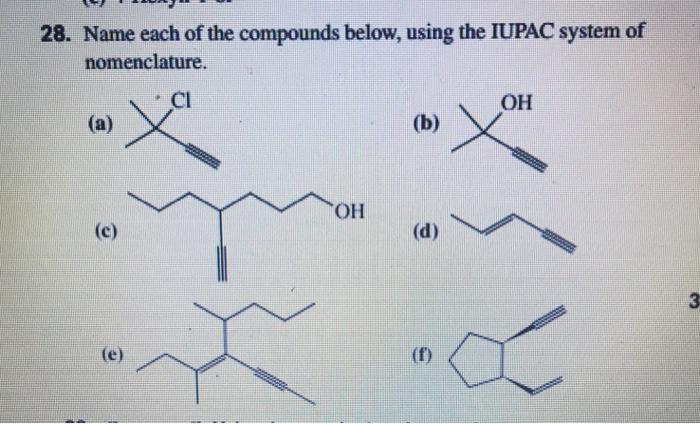
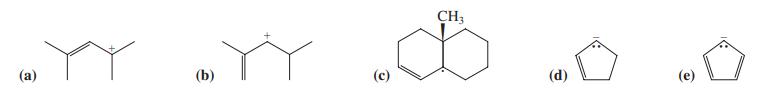
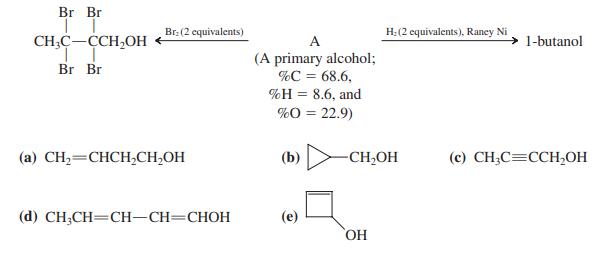
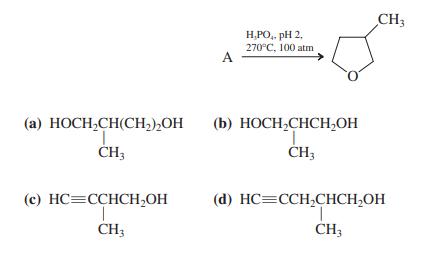
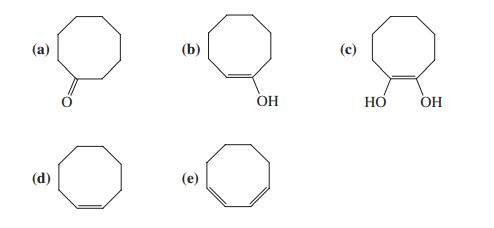
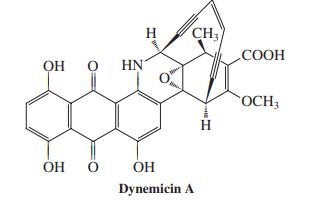
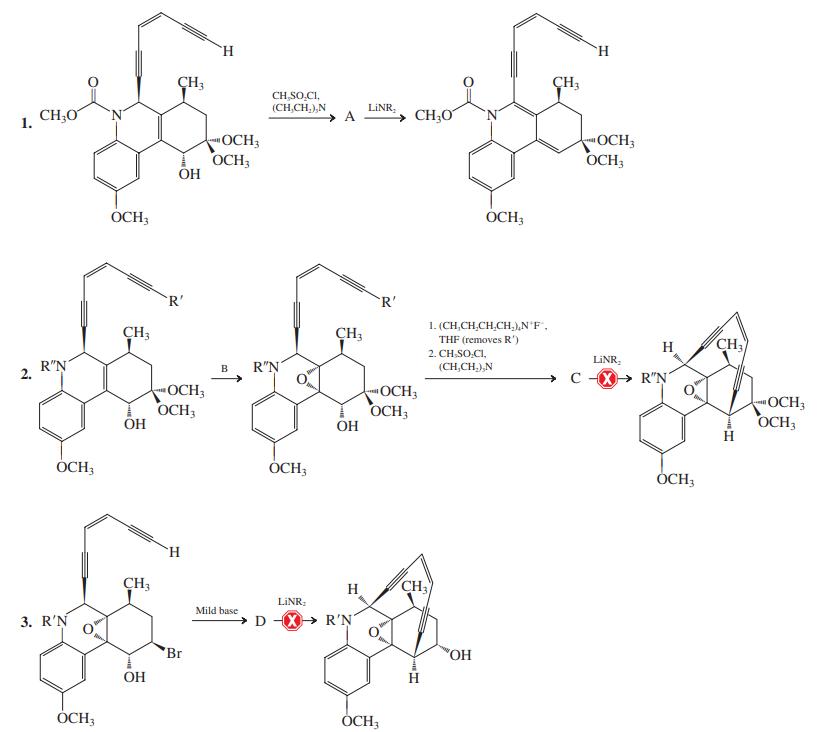
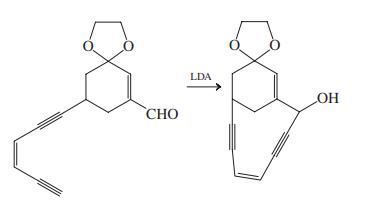
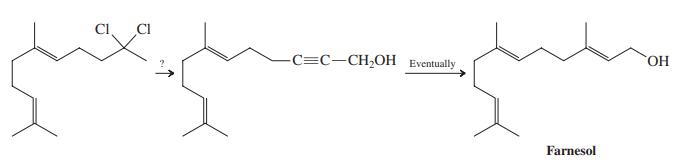
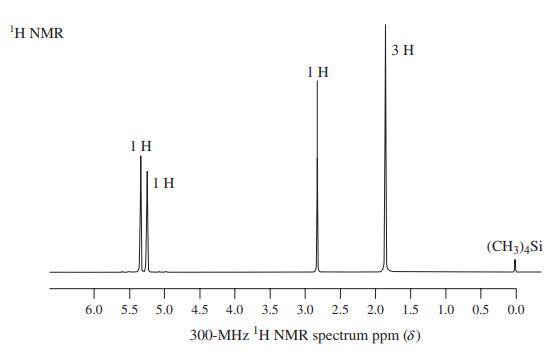
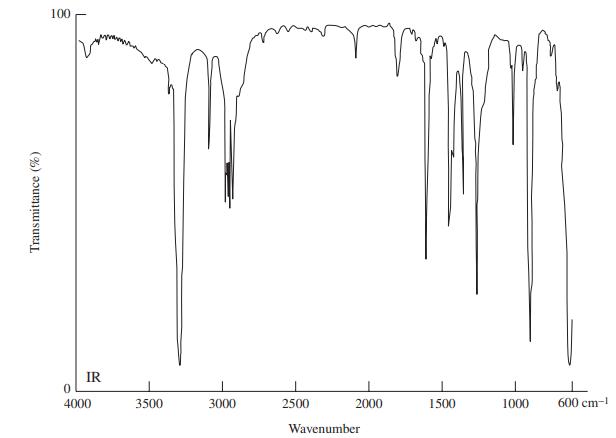
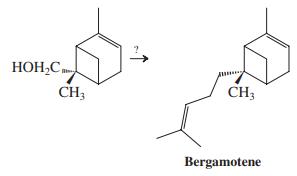
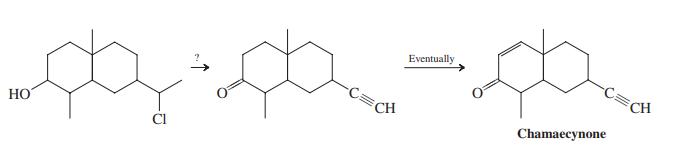
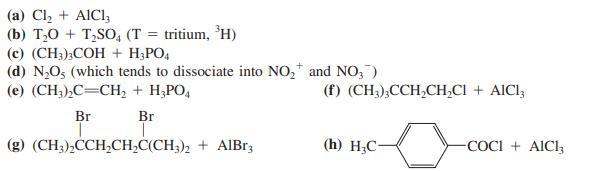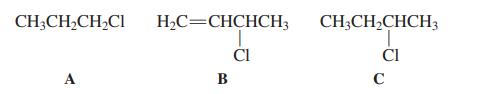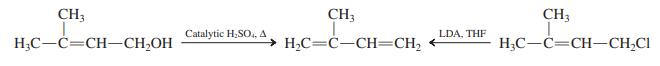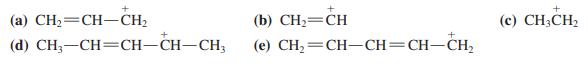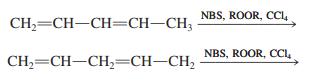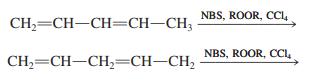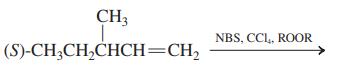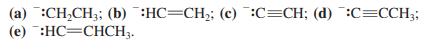![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


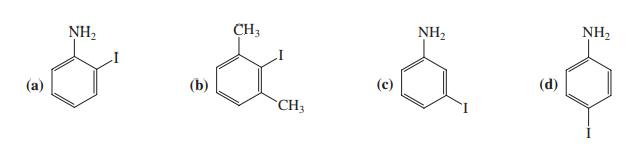

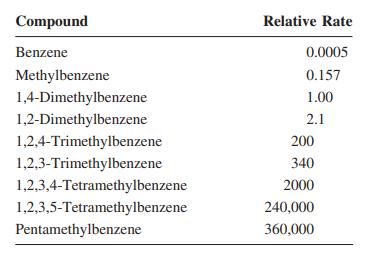
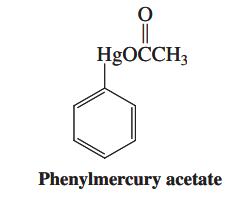
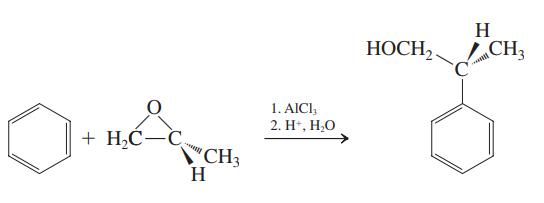
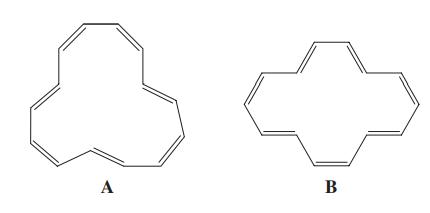
![H H 1,6-Methano[10]annulene](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1589/9/5/2/9965ec4c1e4dfc261589952993368.jpg)