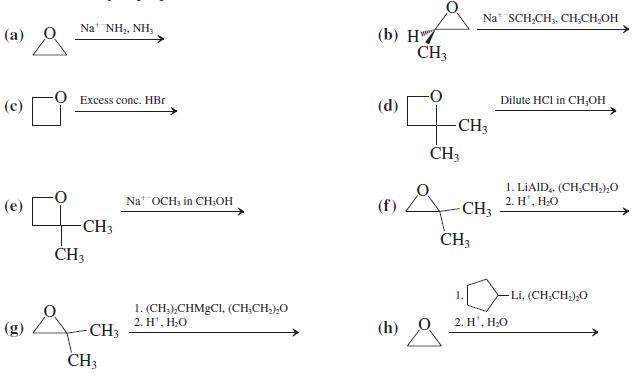
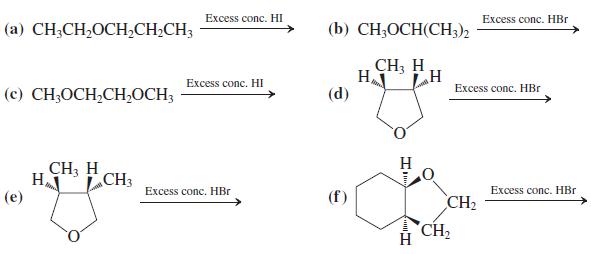
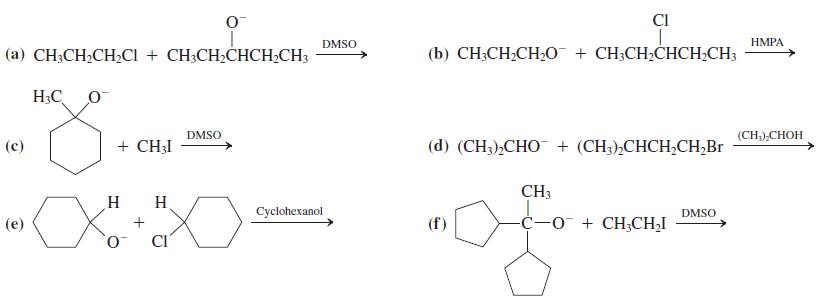
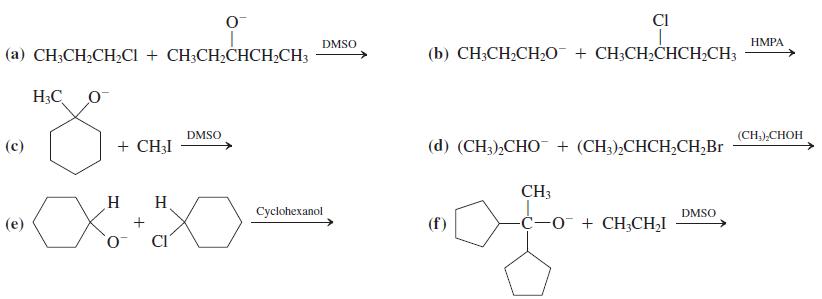
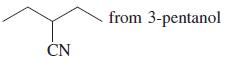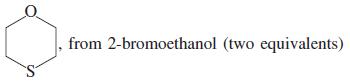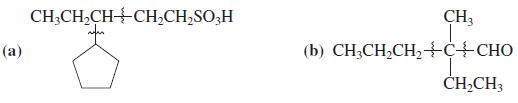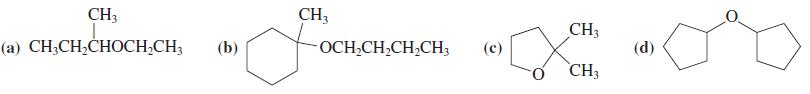![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


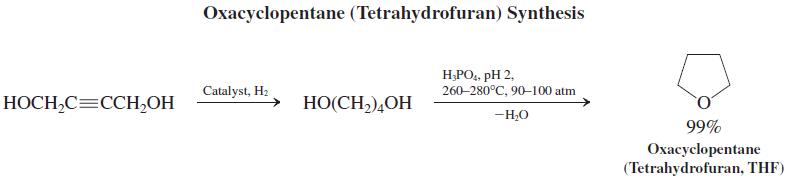
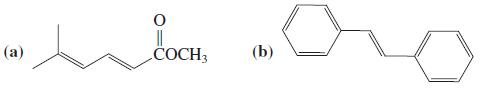
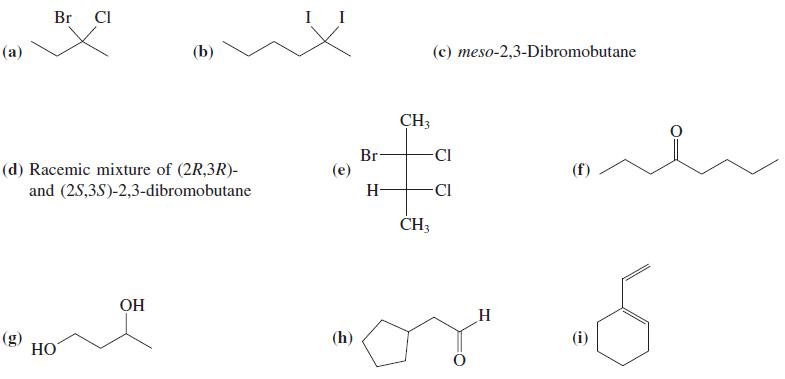
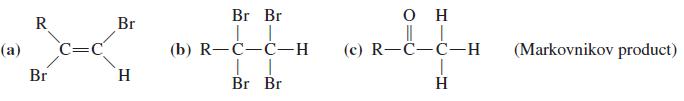
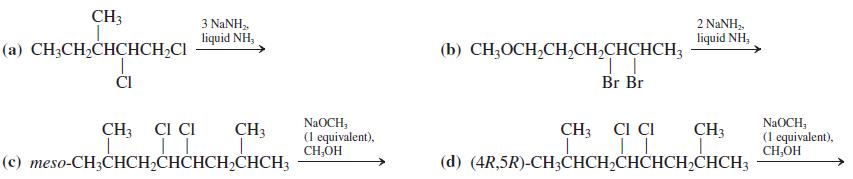
![OH (d) (CH3),CC=CH [Be careful! What is wrong with (CH3);CCI + :C=CH?] (a) (b) (c) ÓH](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1588/5/8/3/7985eafdd76df9e11588583791708.jpg)
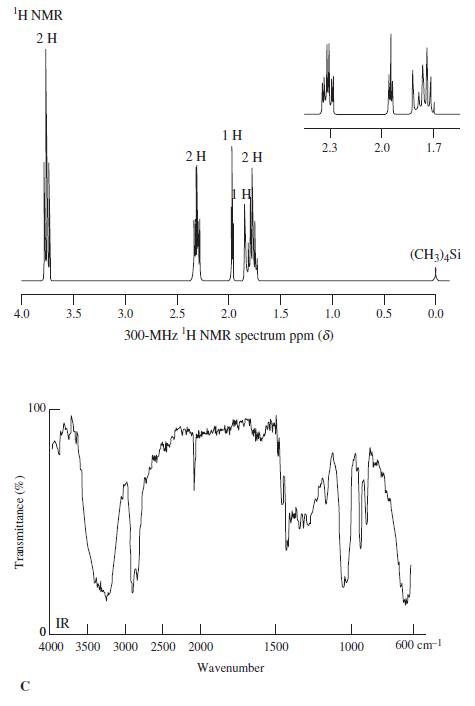
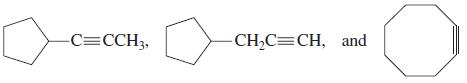
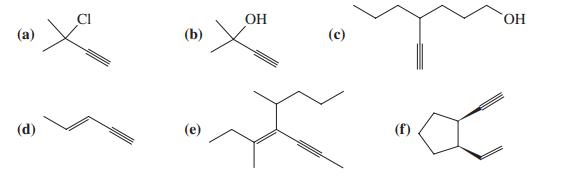
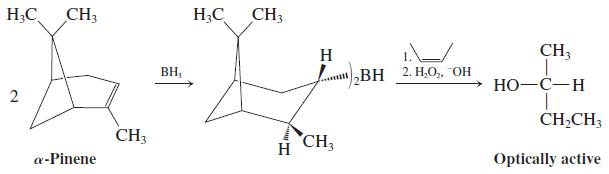

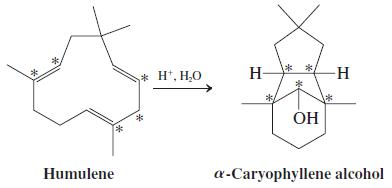
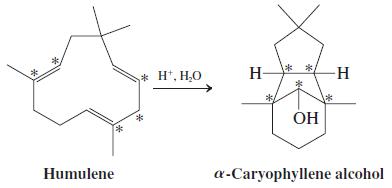
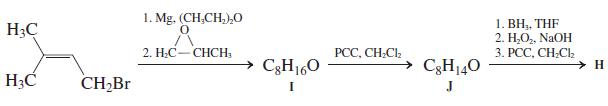
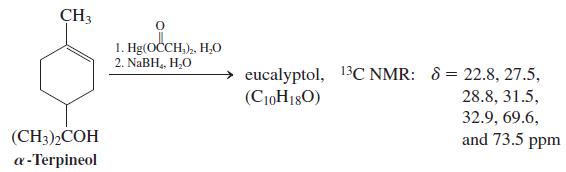
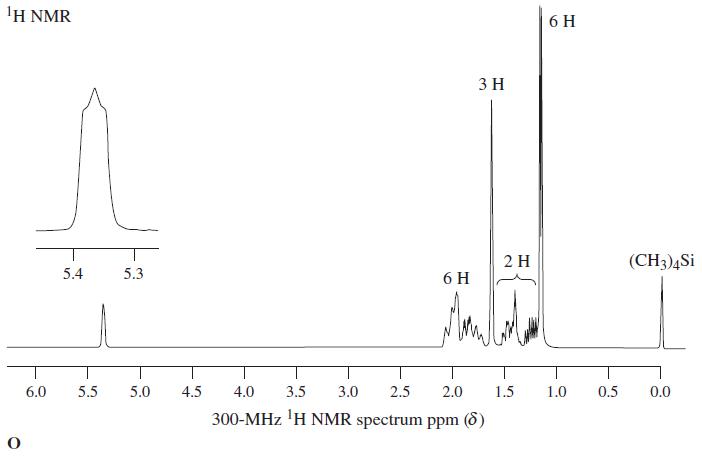
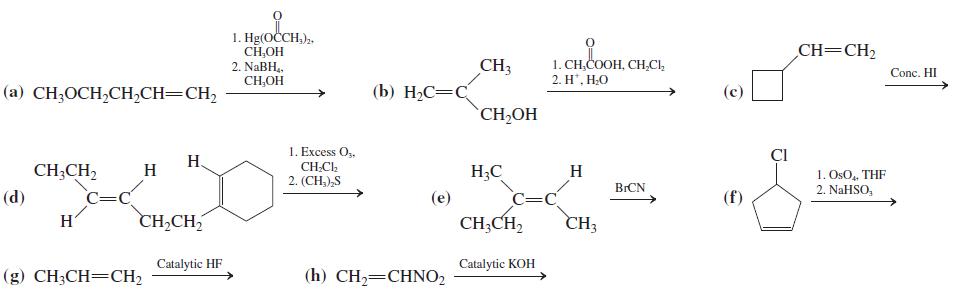
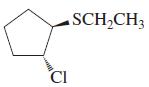
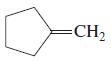
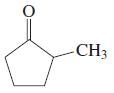
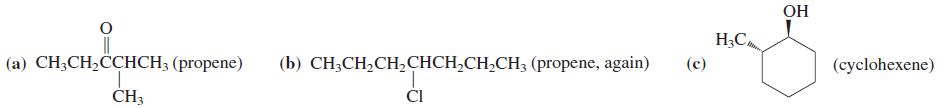
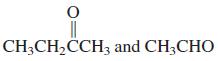
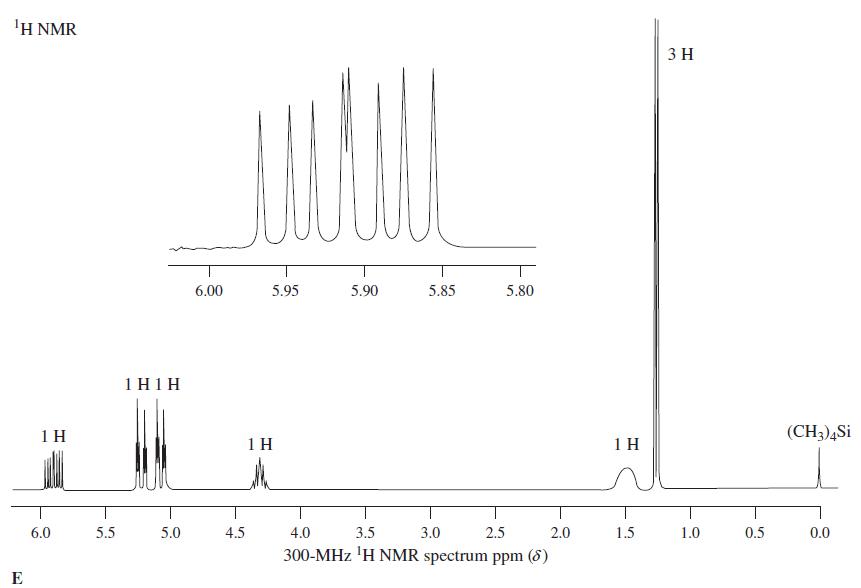
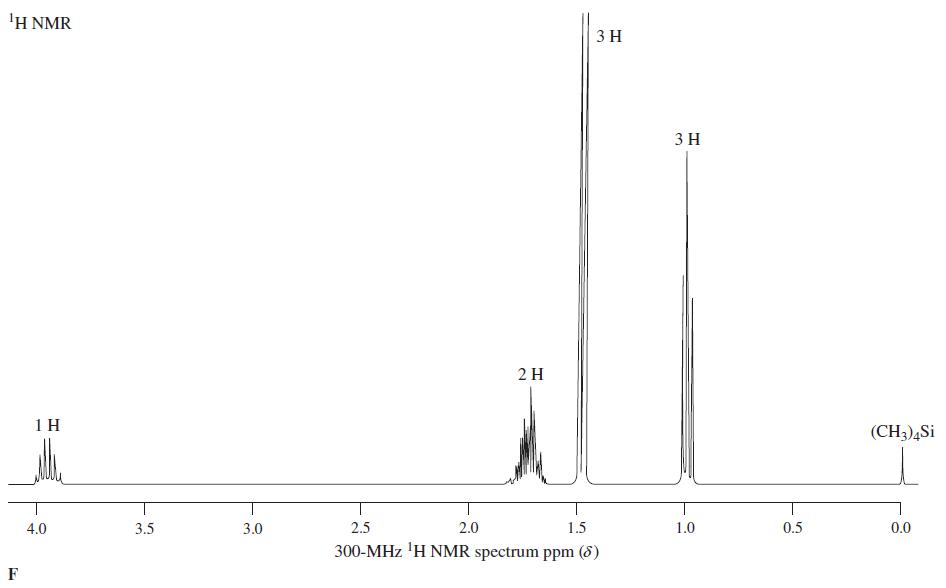
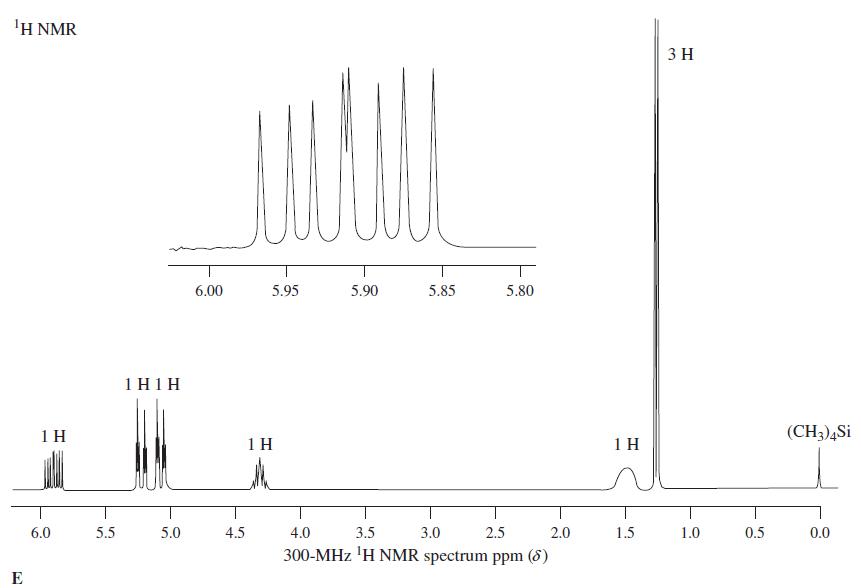
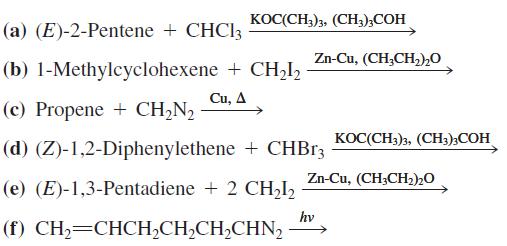
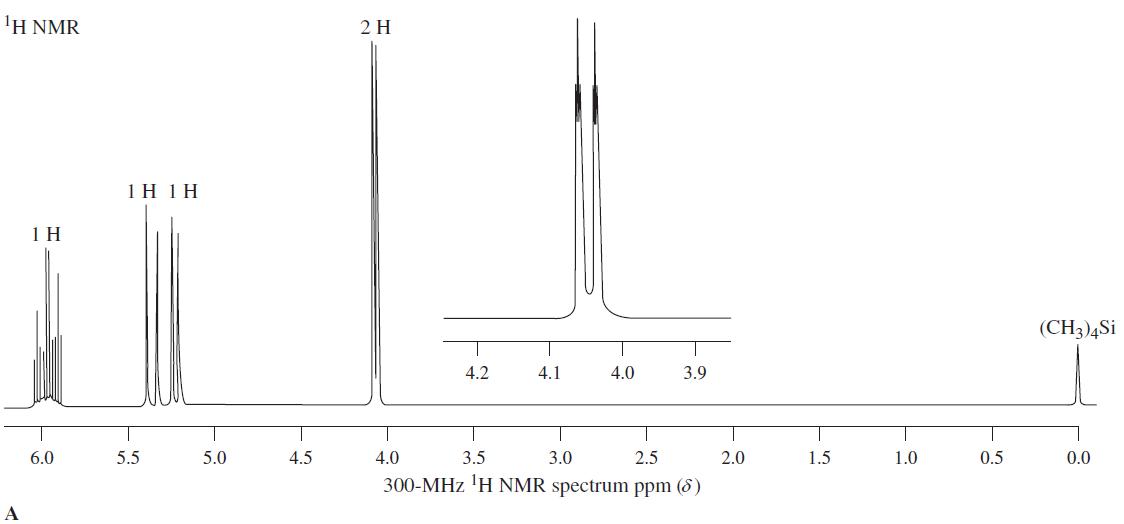
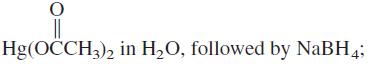
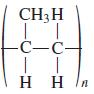
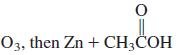
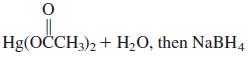
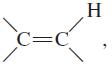
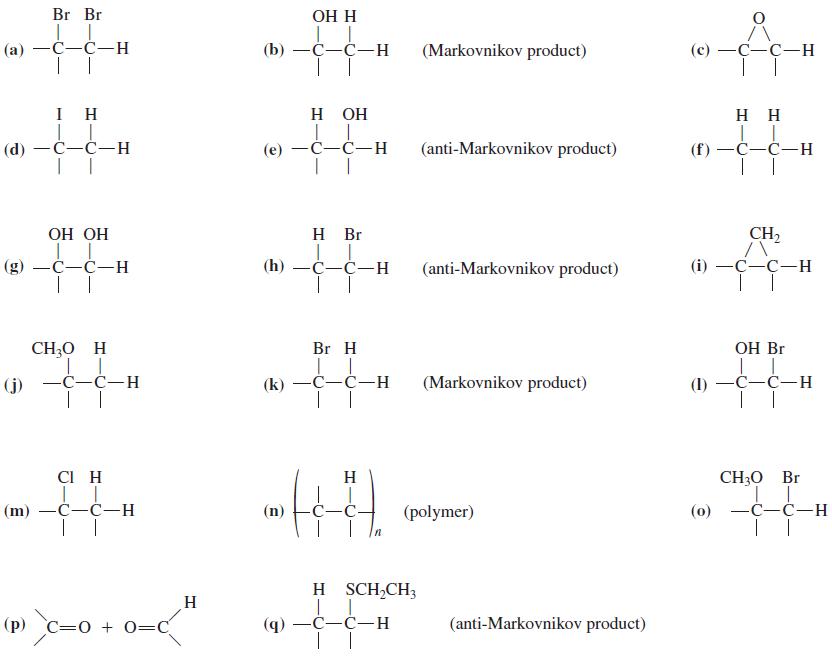
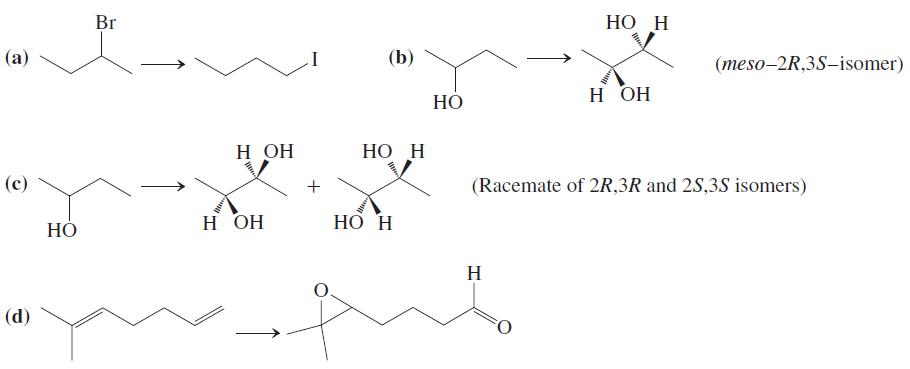
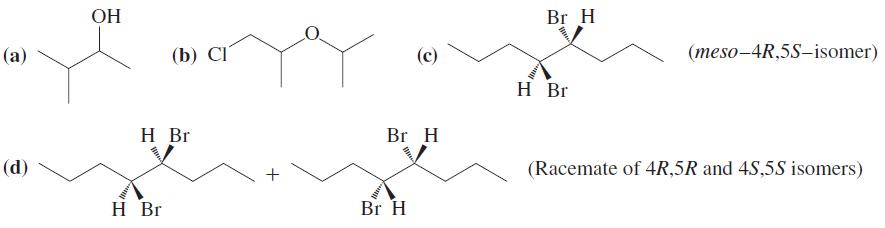
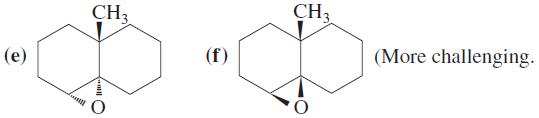
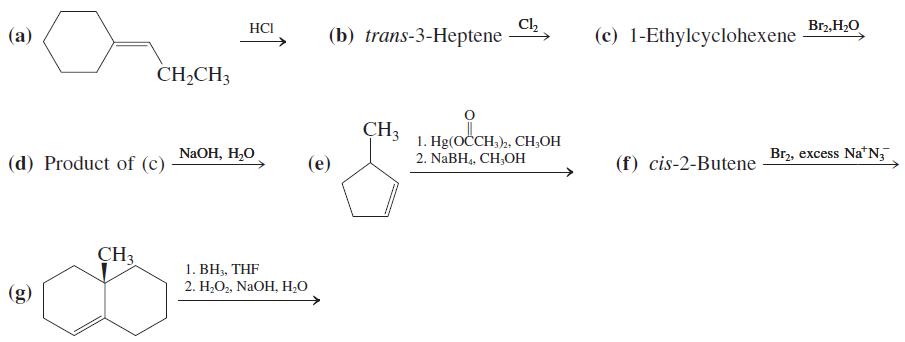
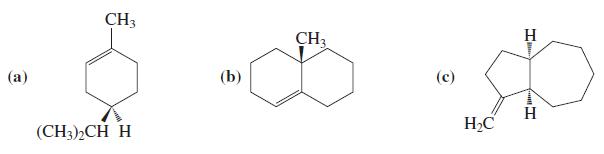
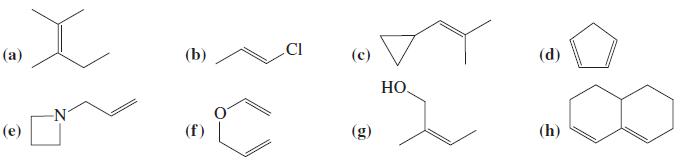
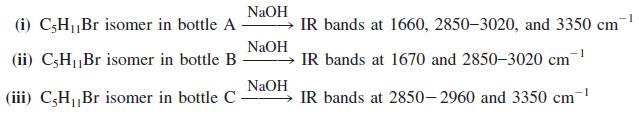
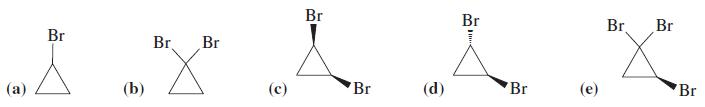
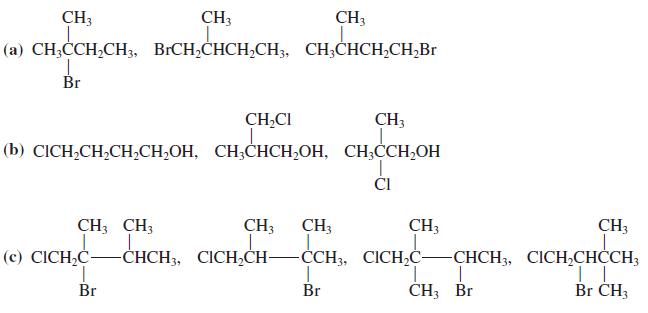
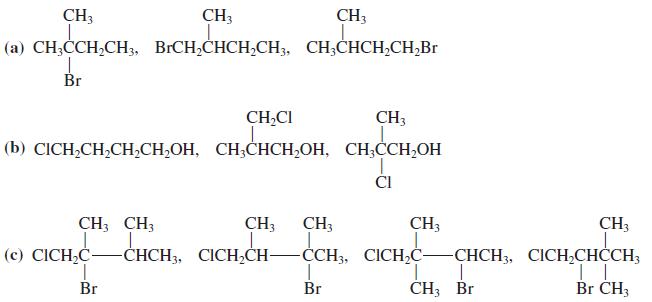
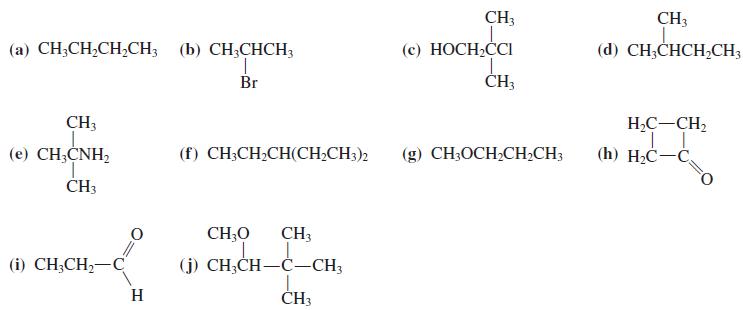
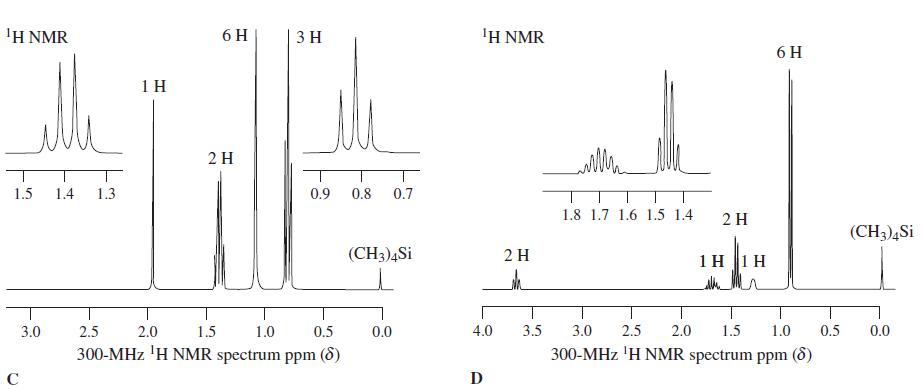
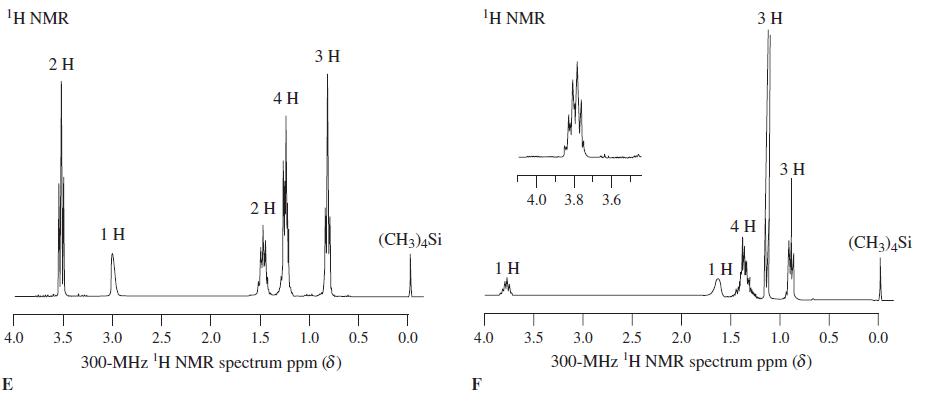
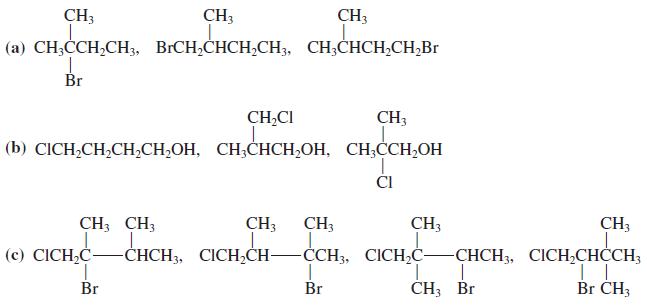
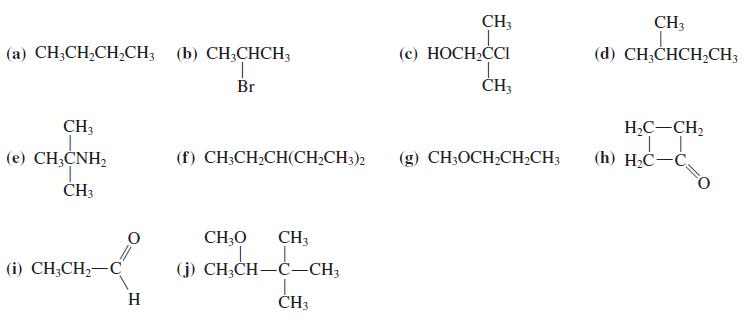
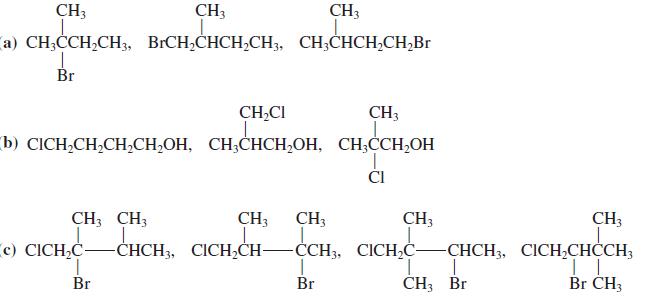
![OH OH A B Fast Slow H;C H;C [Note: Enols are unstable with respect to isomerization to the corresponding ketone (Chapters 13 and 18).] [HO OH Fast C,H C,H Enol OH Slow](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1588/2/3/8/5365eaa98c8a47af1588238531725.jpg)