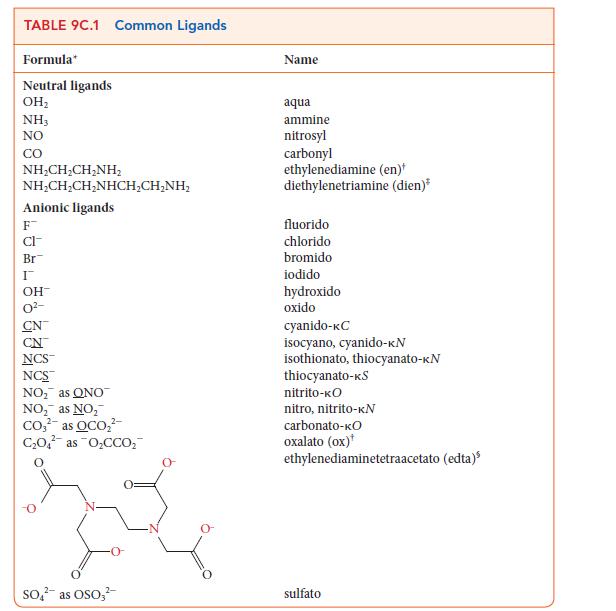
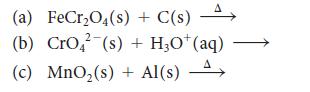![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


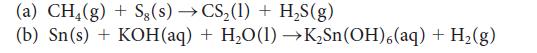
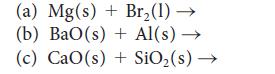
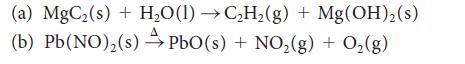
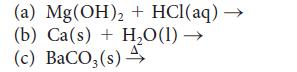
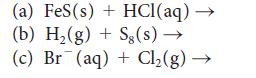
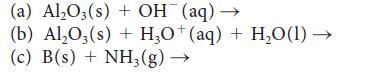
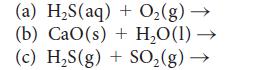
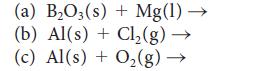
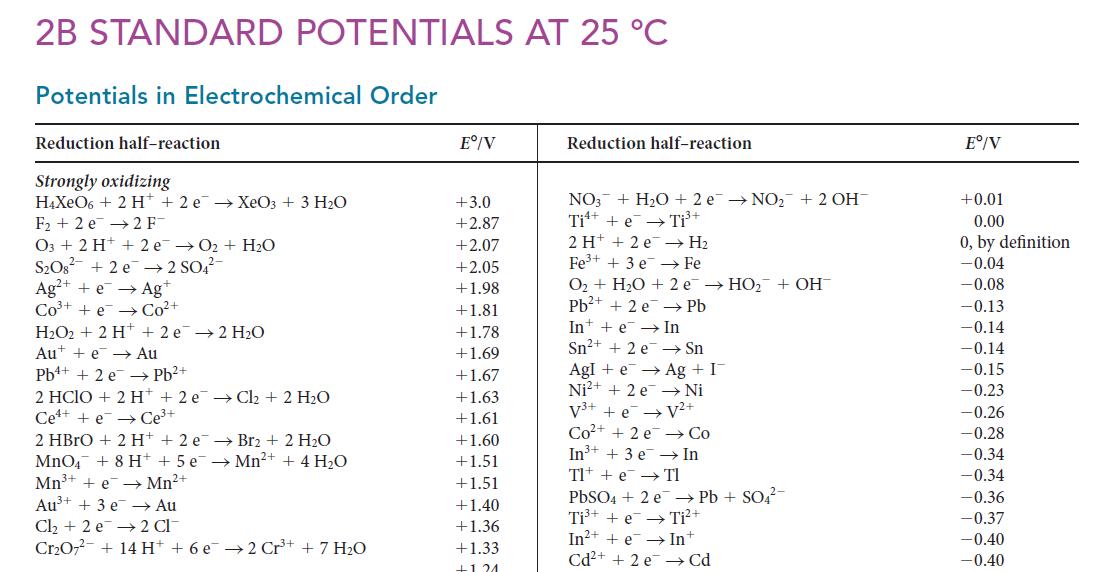
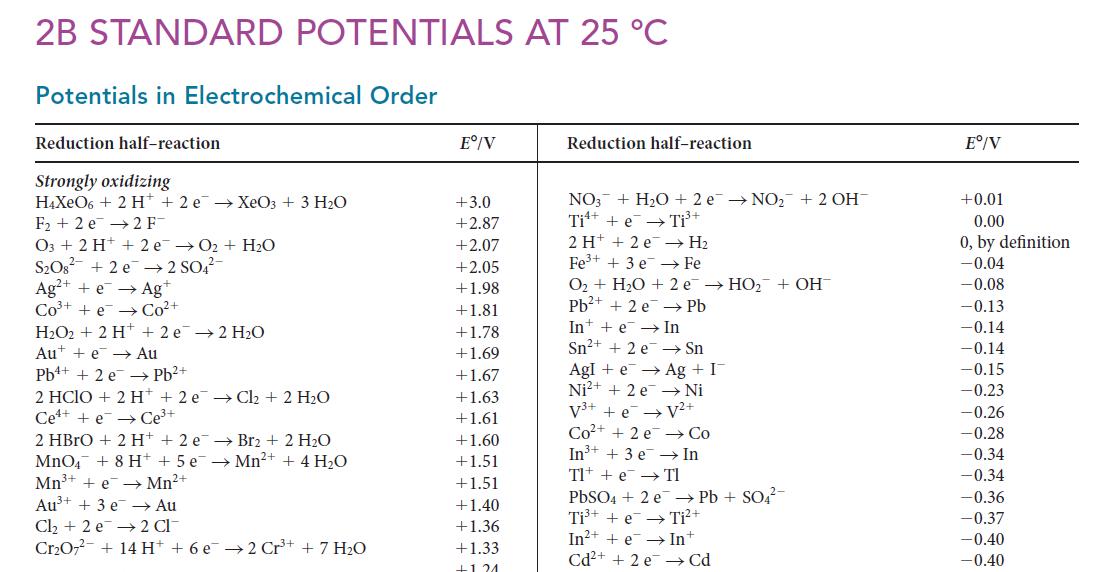
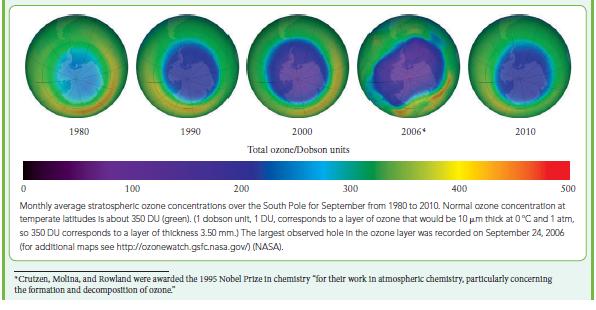
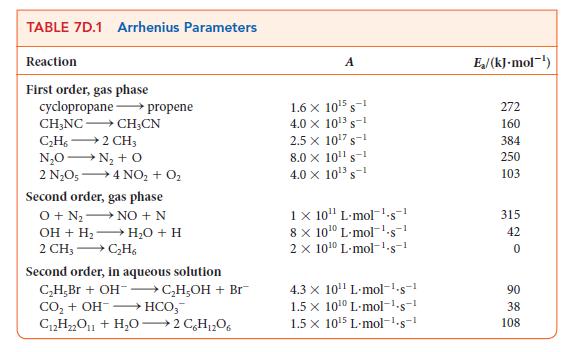
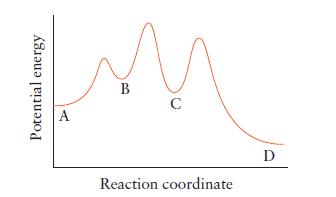
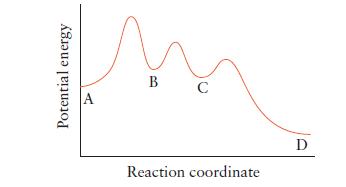
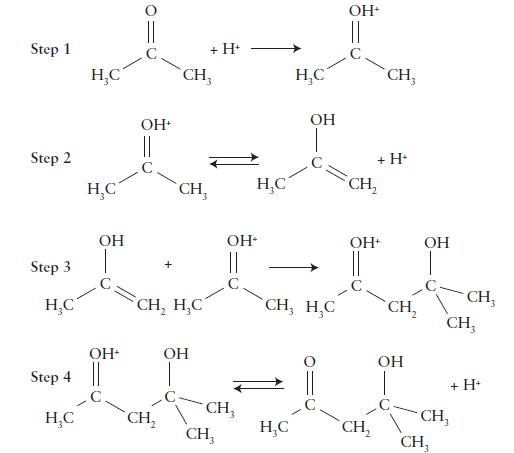
![(a) Rate = k[CH3CHO] (Products are CH3 and CHO.) (b) Rate = k,[1][Ar] (Products are 1 and Ar; the role of the](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/0/8/2/96765923e176e6541704082966937.jpg)
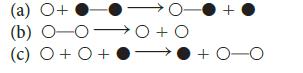
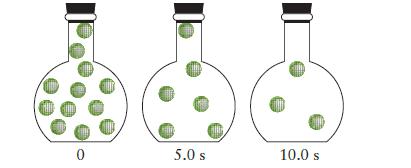
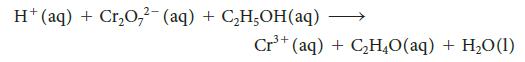
![Fe+ (aq) + SCN(aq) Fe(SCN)+ (aq) 2+ Kf= [Fe(SCN)+] [Fe+][SCN]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/1/0/3/85265928facd2c731704103851944.jpg)