![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


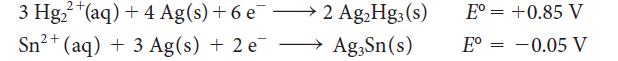
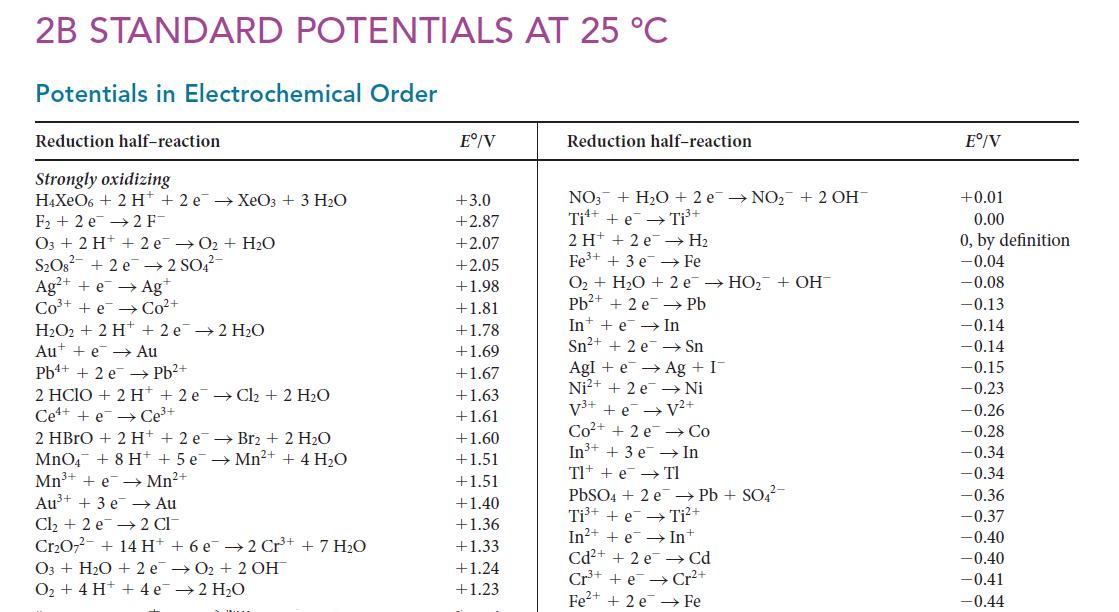
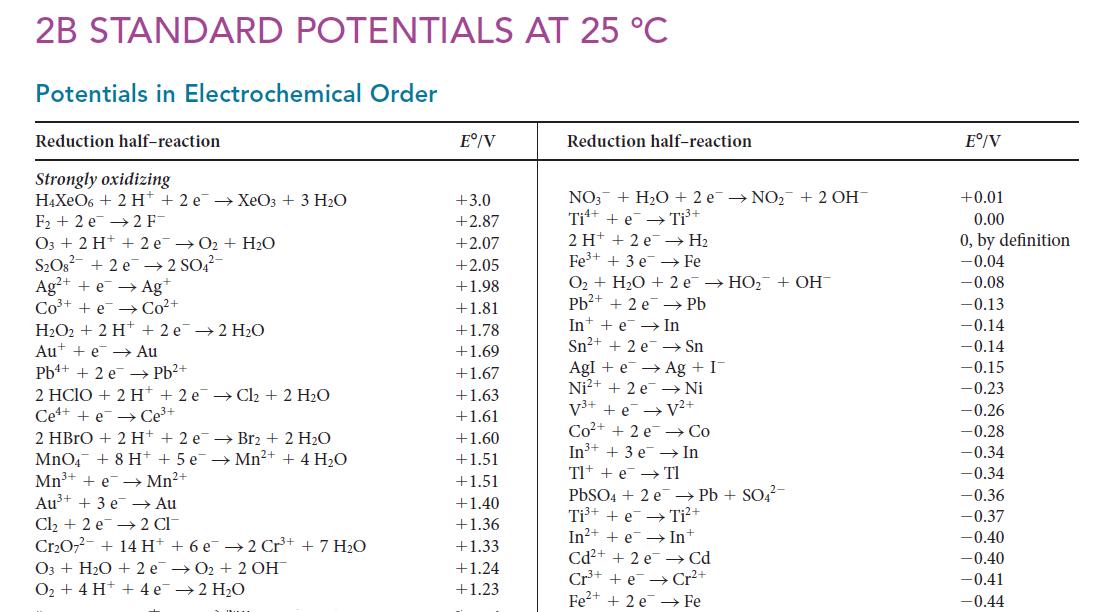
![Average rate of formation of P = A[P] At (1b)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/3/3/147658ff4db9fd501703933145120.jpg)
![2 03(g) 3 0(g) [03] [0] Rate of decomposition of O3 = k,-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/4/399659020cf2669b1703944398822.jpg)
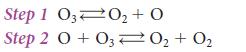
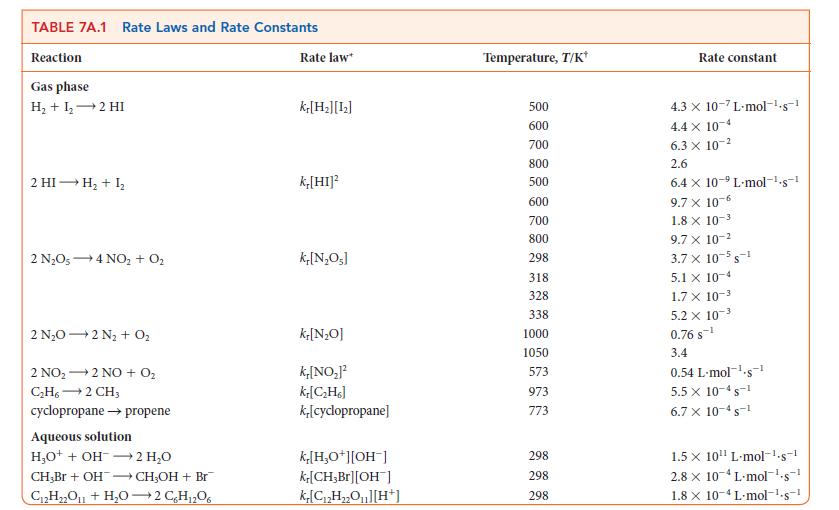
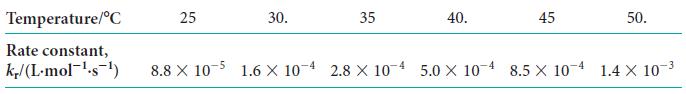
![Experiment 1234 4 Initial concentration, [J]/(mol.L-) BrO3 0.10 0.20 0.10 0.20 Br 0.10 0.10 0.30 0.10 H3O+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/3/3/272658ff55825c4b1703933269798.jpg)
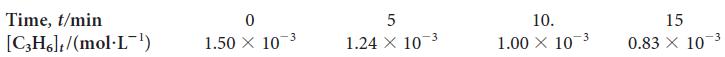
![[A], [A]o [A] = [A]oe In -k,t -ket (la) (1b)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/0/2036590106be56ef1703940203735.jpg)

![Time, t/s [HI]/(mmol-L-) 0. 10.0 1000. 2000. 3000. 4000. 5000. 4.4 2.8 2.1 1.6 1.3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/3/4/371658ff9a4003c41703934369042.jpg)
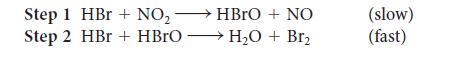
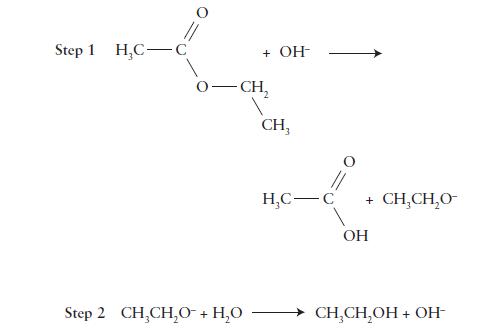
![Time, t/s [NO5]/(mmol-L-) 0. 2.15 1.11 1.88 2.22 1.64 3.33 4.44 1.43 1.25](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/3/4/732658ffb0cd1b1c1703934731134.jpg)
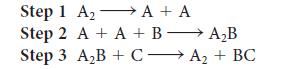
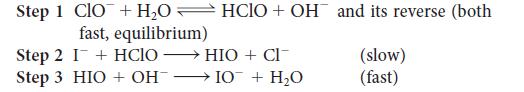
![Rate of formation of product = k [E]o[S] KM + [S] (1a)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/5/1/94965903e4d2fbd11703951946570.jpg)
![Time, t/s [HI]/(mol.L-) 0 1.0 1000. 2000. 0.11 0.061 3000. 4000. 0.041 0.031](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/2/611659019d333e2a1703942607413.jpg)
![Time, t/s [1]/(mmol.L-) 0 1.00 1.0 0.43 2.0 0.27 3.0 4.0 0.20 0.16](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/2/85465901ac6c04481703942854536.jpg)