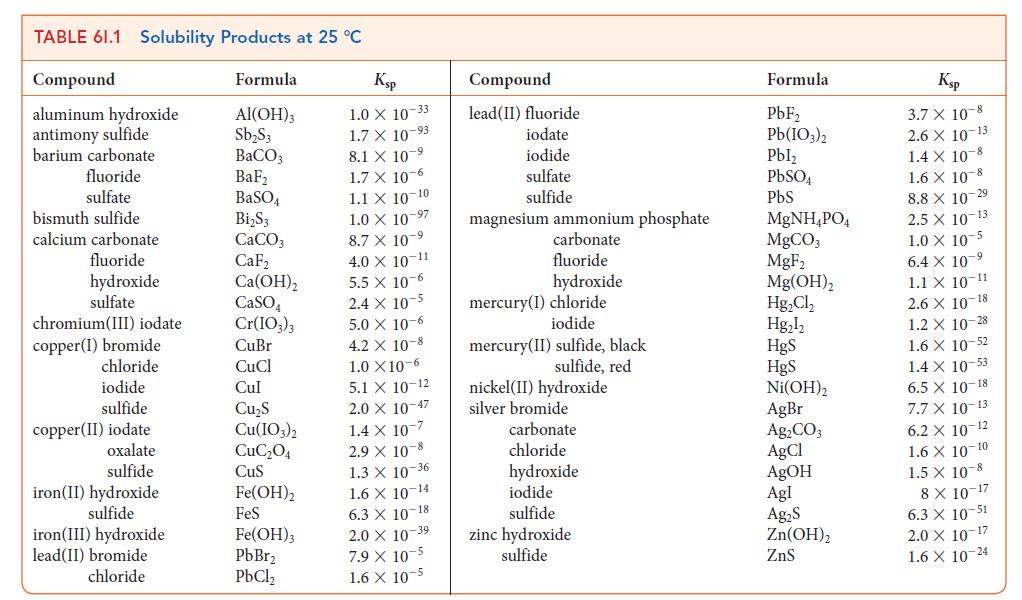
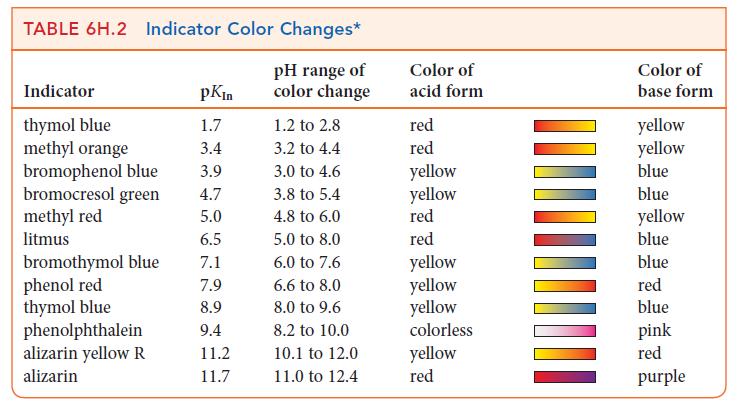
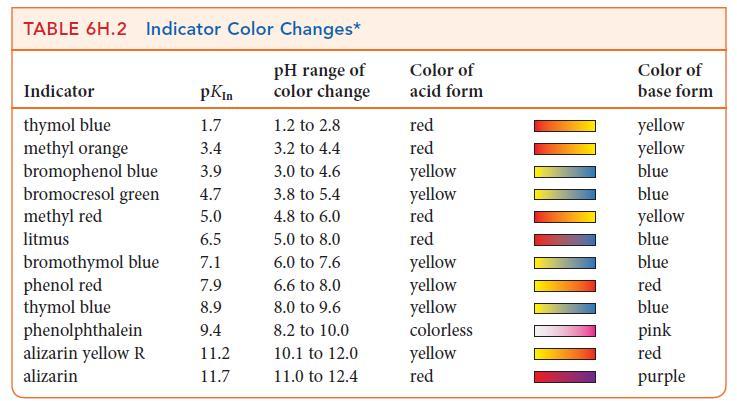
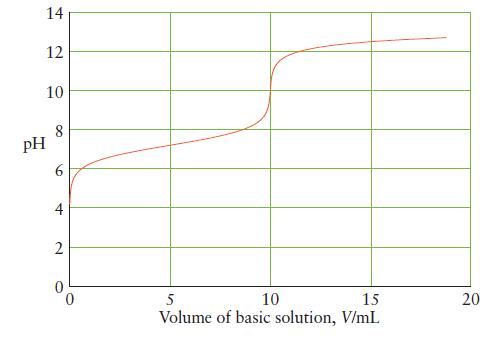
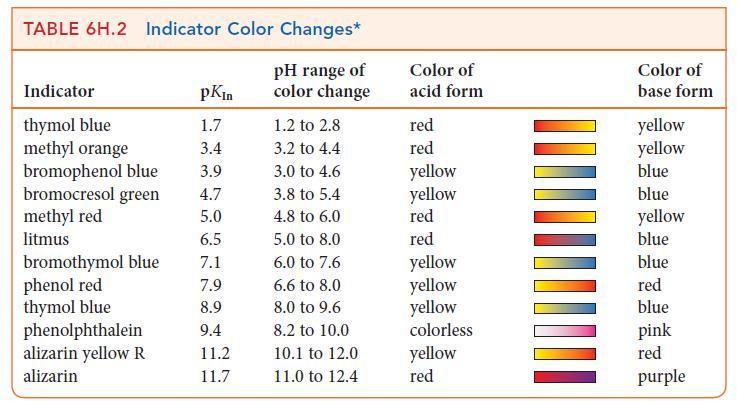
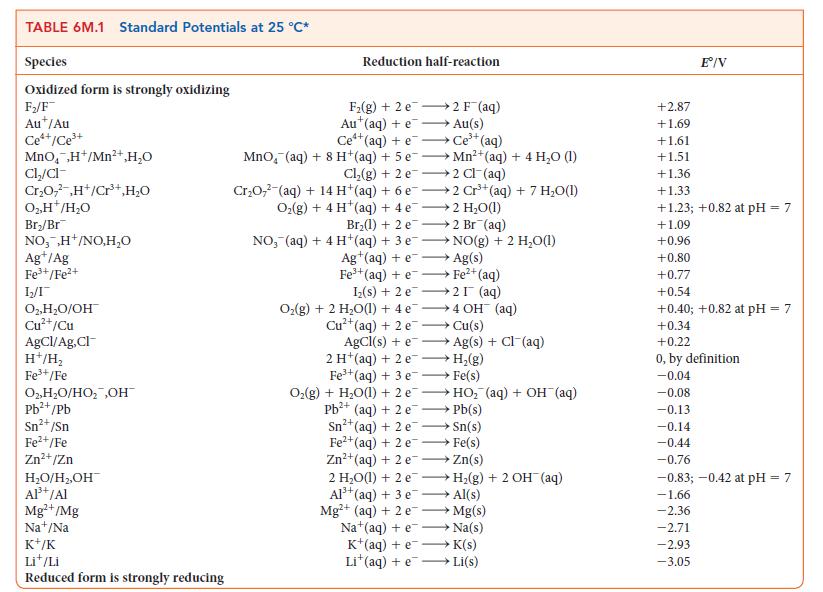
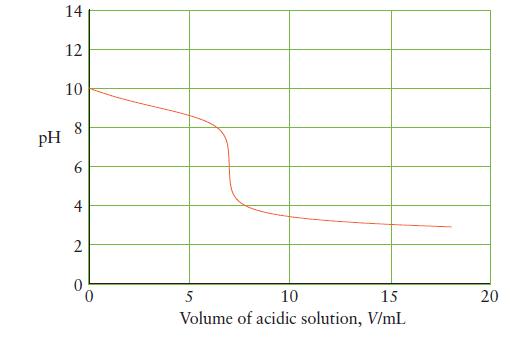
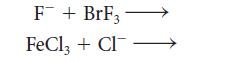![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


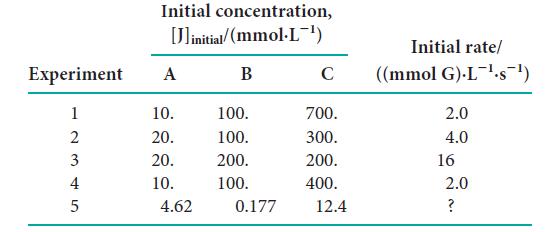
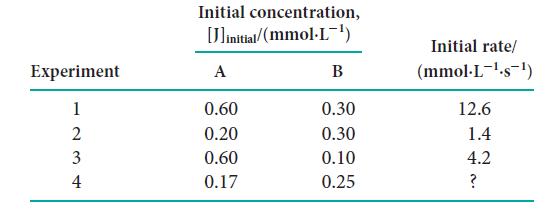
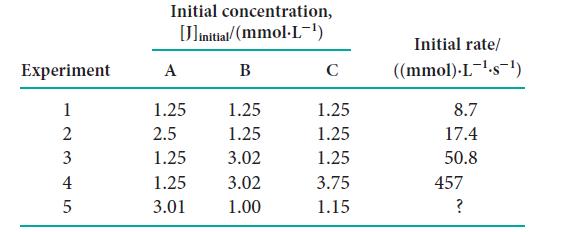
![Experiment 123 + 2 4 Initial concentration, [J]initial/(mmol.L-) A 1.72 3.44 1.72 2.91 B 2.44 2.44 0.10 1.33](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/3/5/808658fff4040c1a1703935805719.jpg)