![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


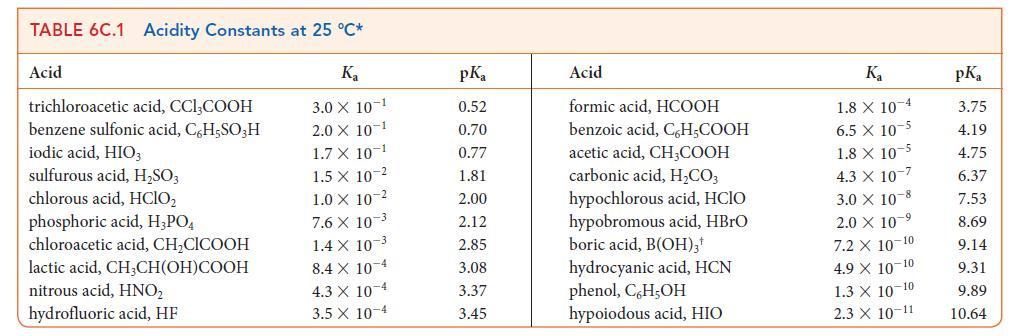
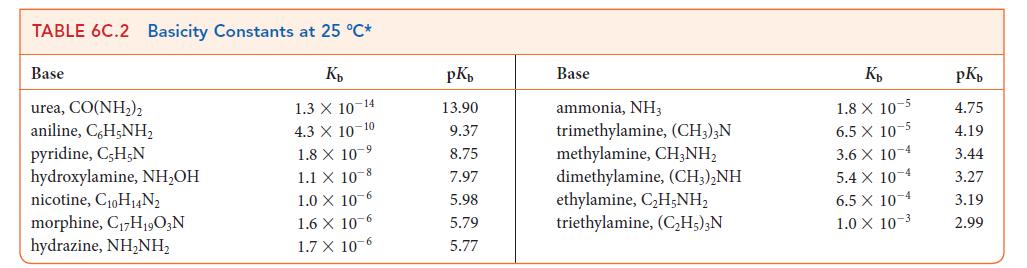
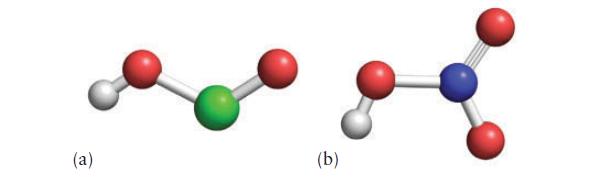
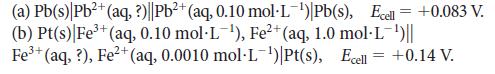
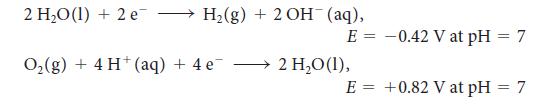
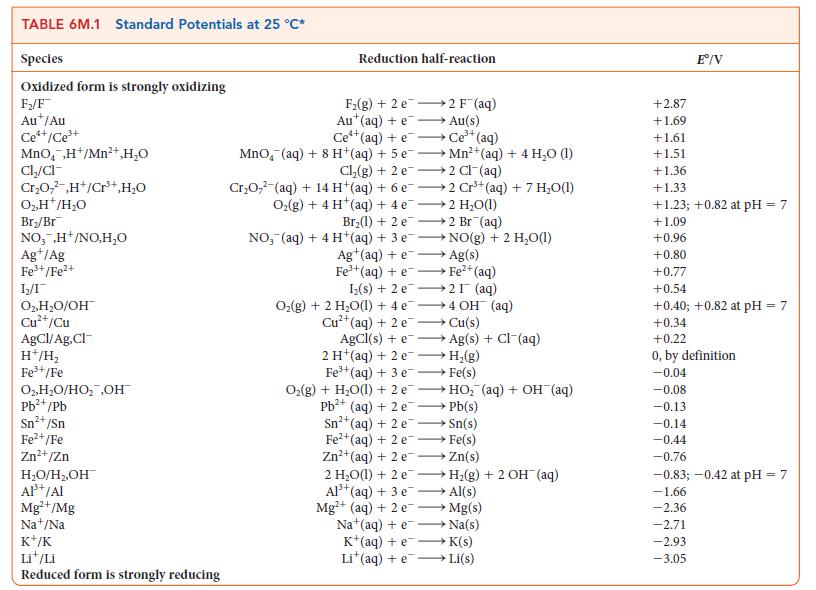
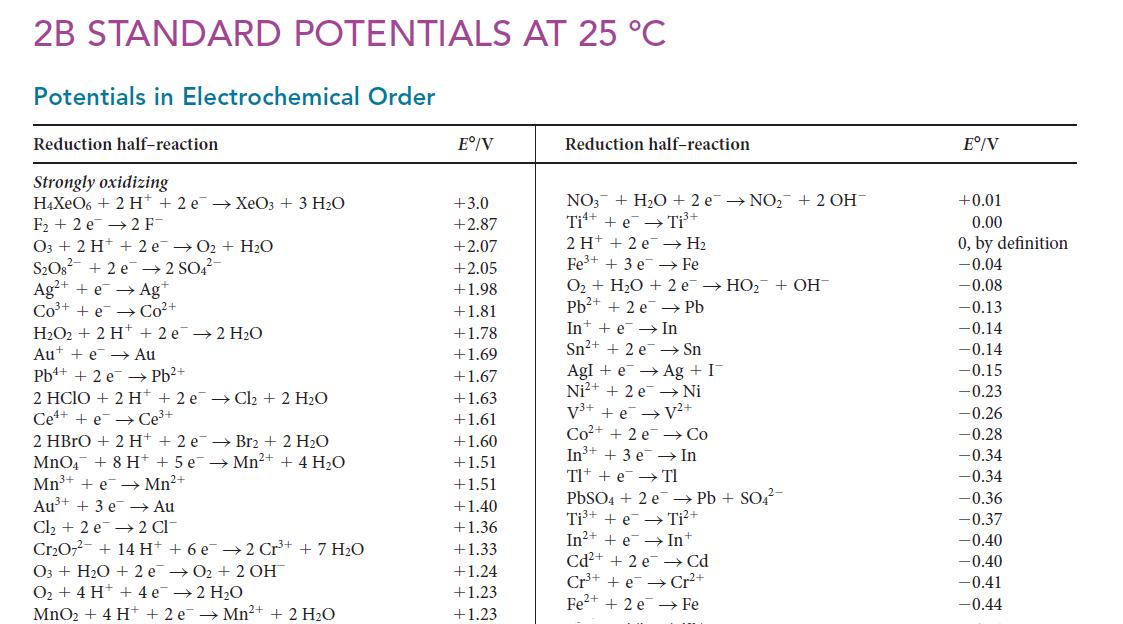
![[H3O+] (i) 1.50 mol-L- (ii) (iii) (iv) [OH-] 1.50 mol-L- PH 0.75 POH 0.75](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/7/7/1/403658d7d0b6d0db1703771404326.jpg)
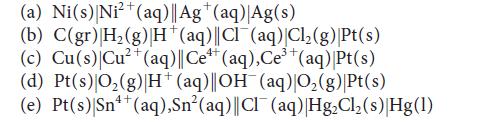
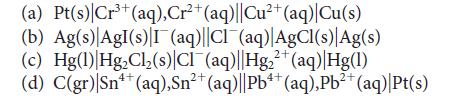
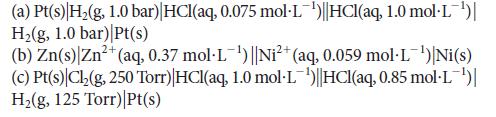
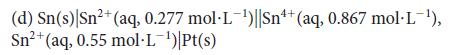
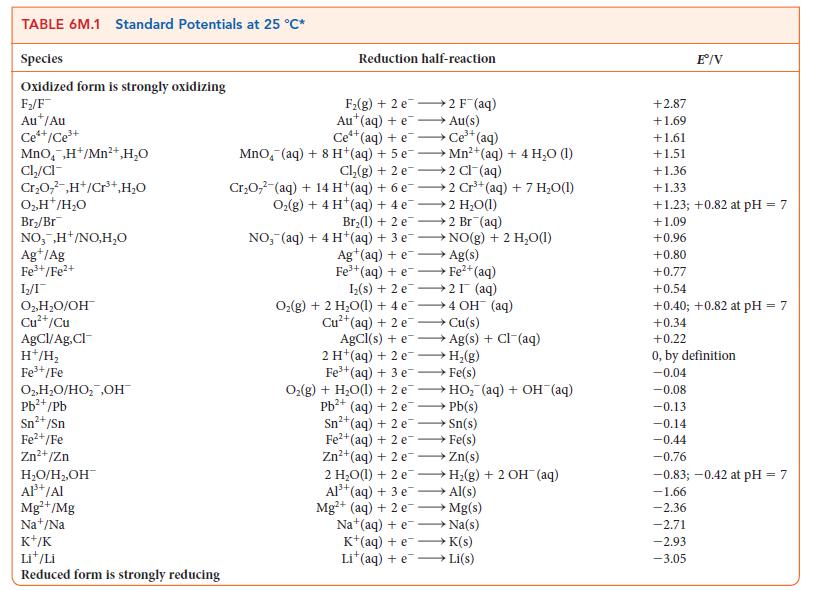
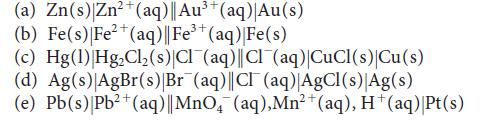
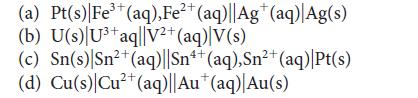
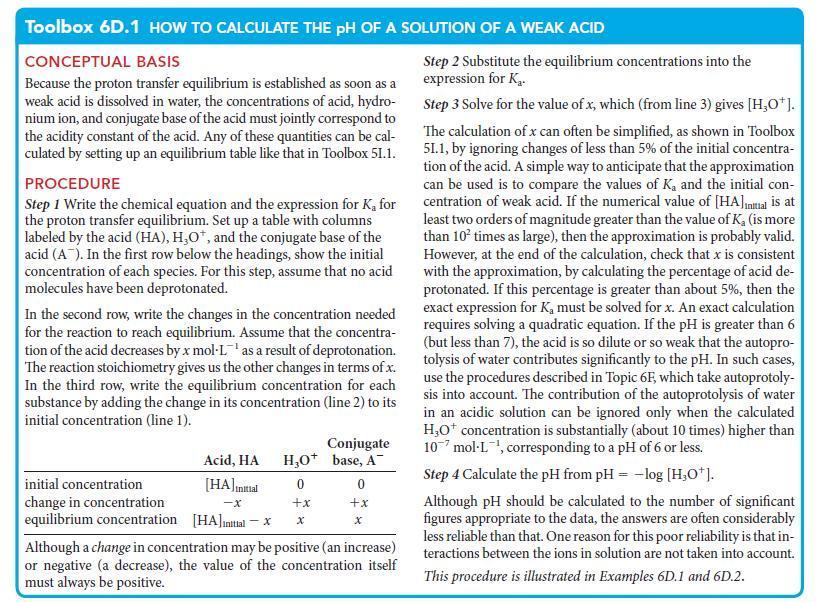
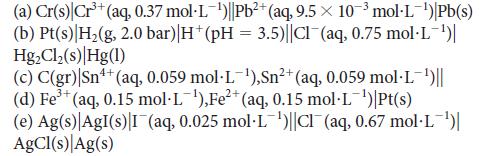
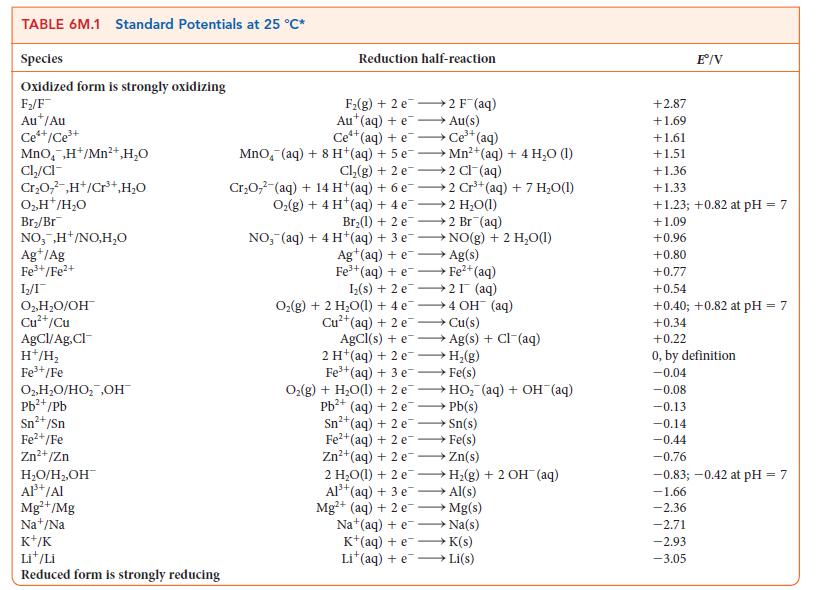
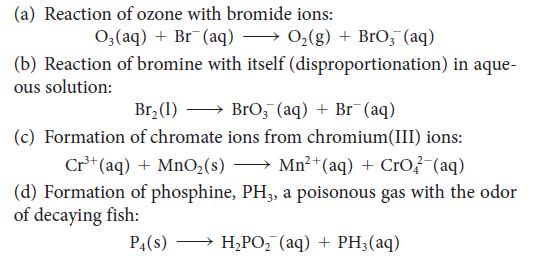
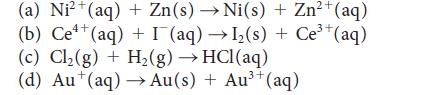
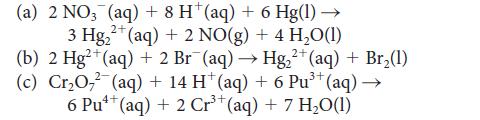
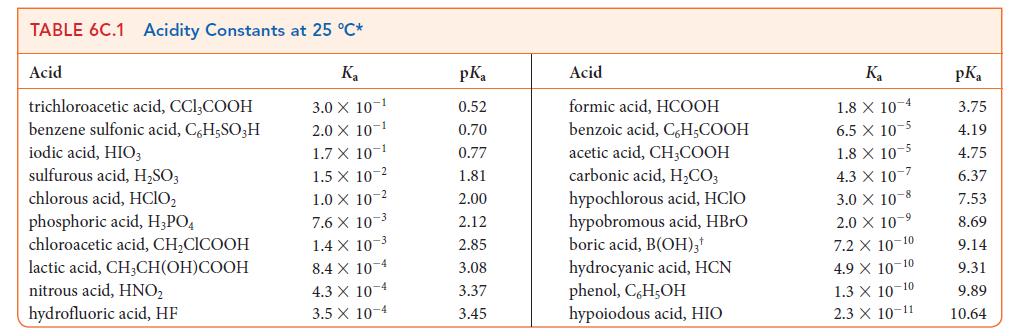
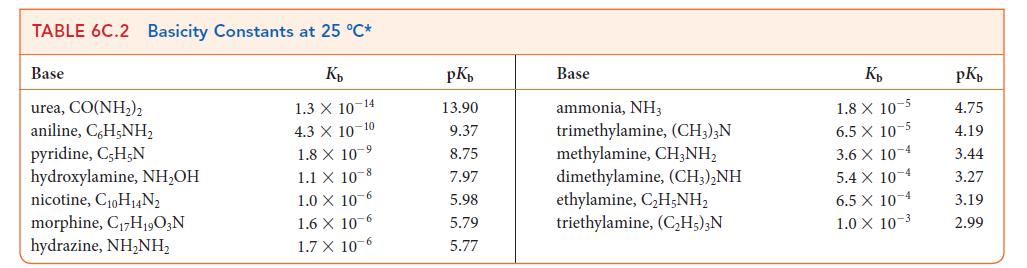
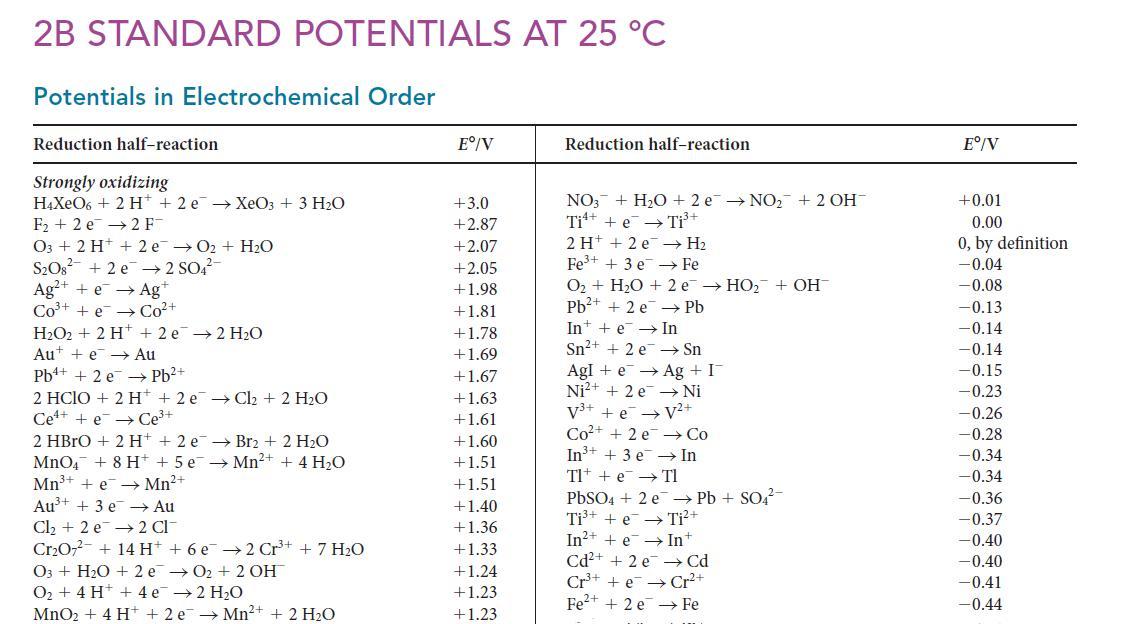
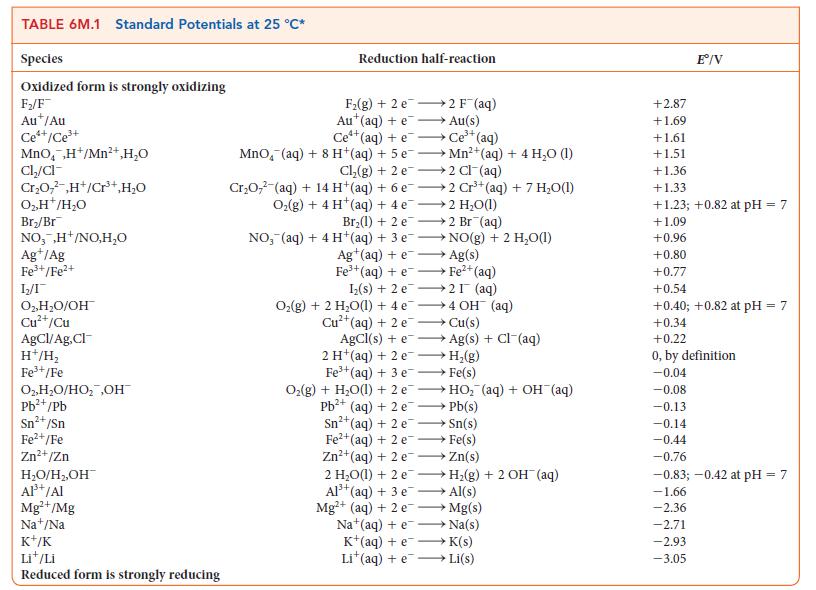
![[H3O+] (i) 0.50 mol-L- (ii) (iii) (iv) [OH-] -1 0.50 mol-L- pH -0.10 POH -0.10](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/7/7/1/414658d7d16b86221703771415926.jpg)