![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


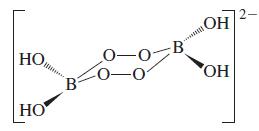
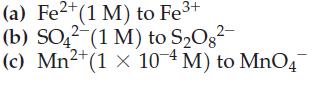
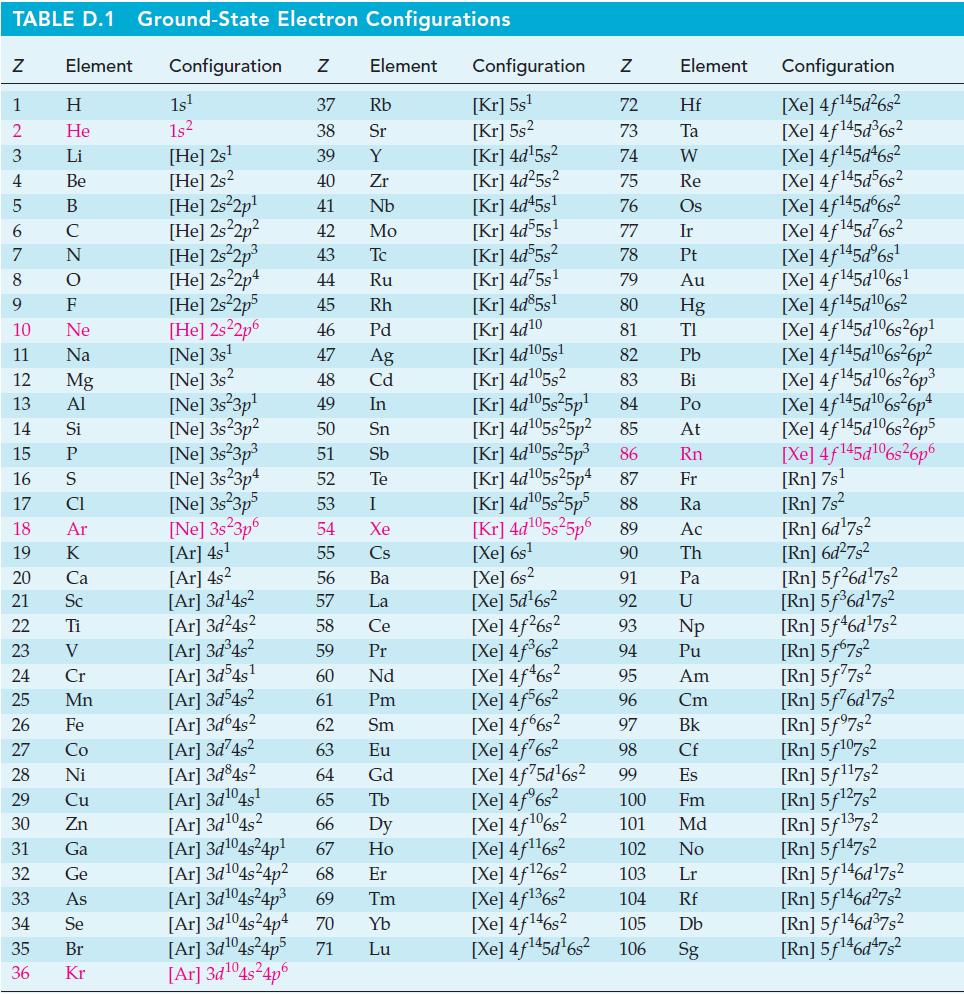
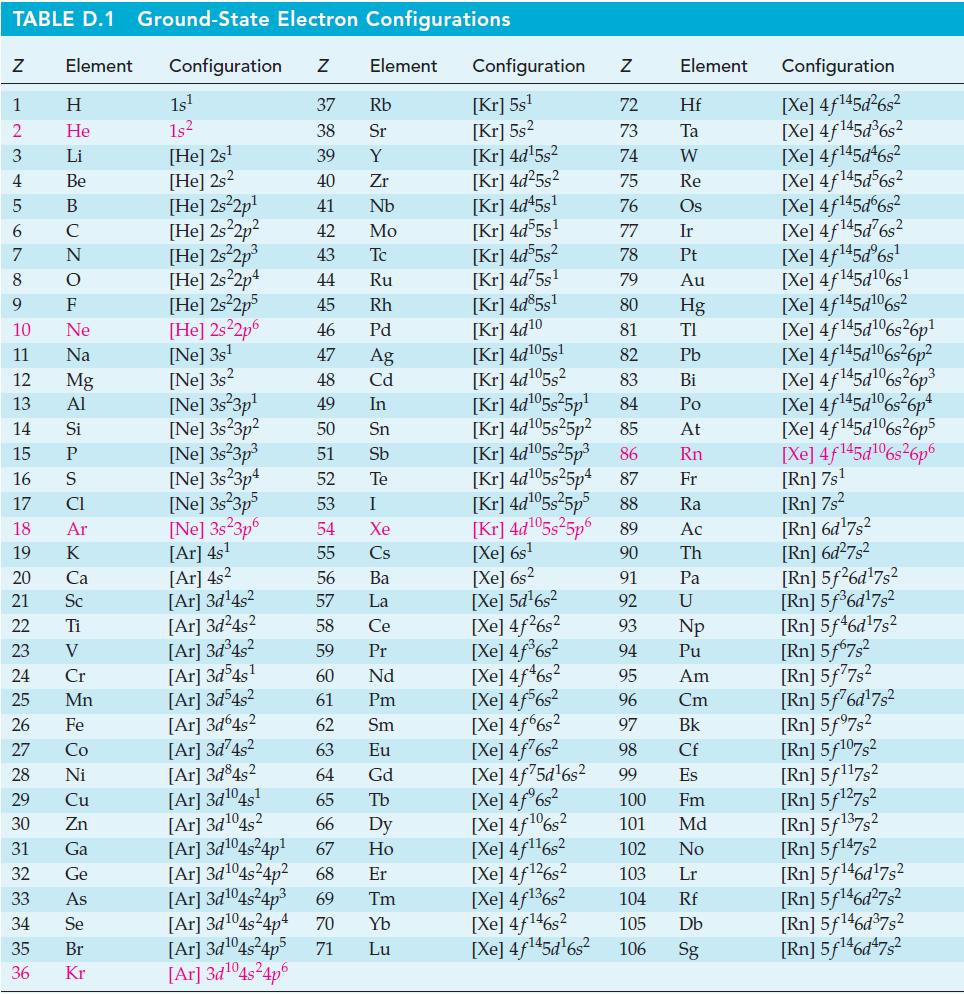
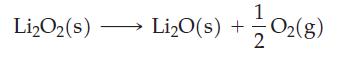

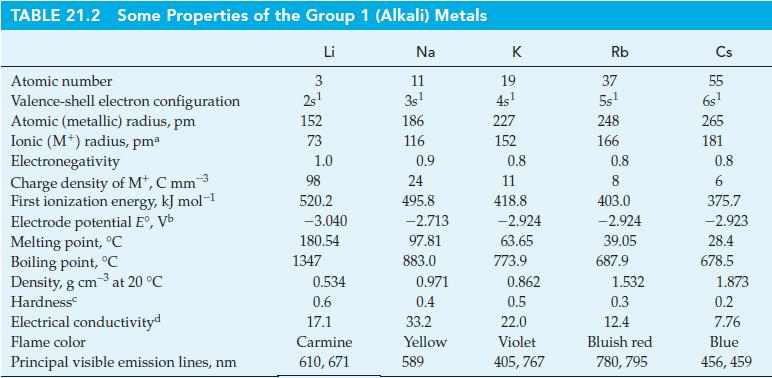
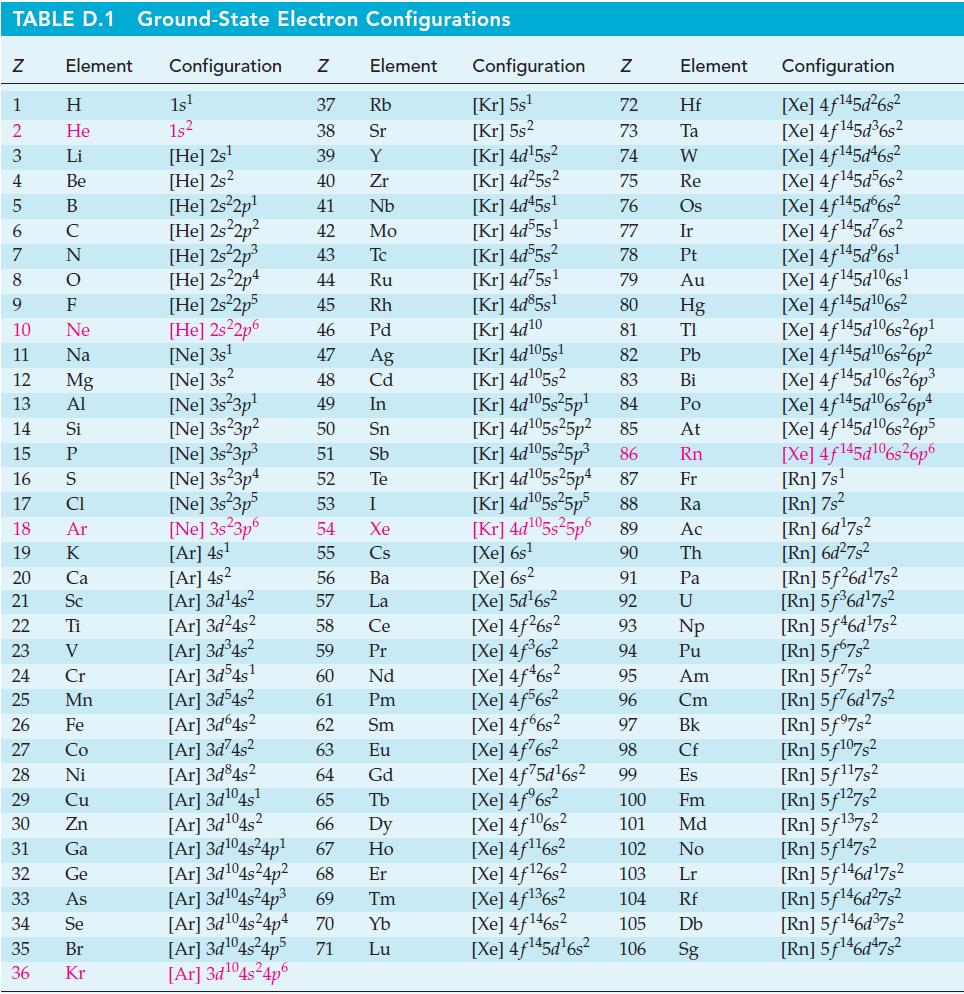
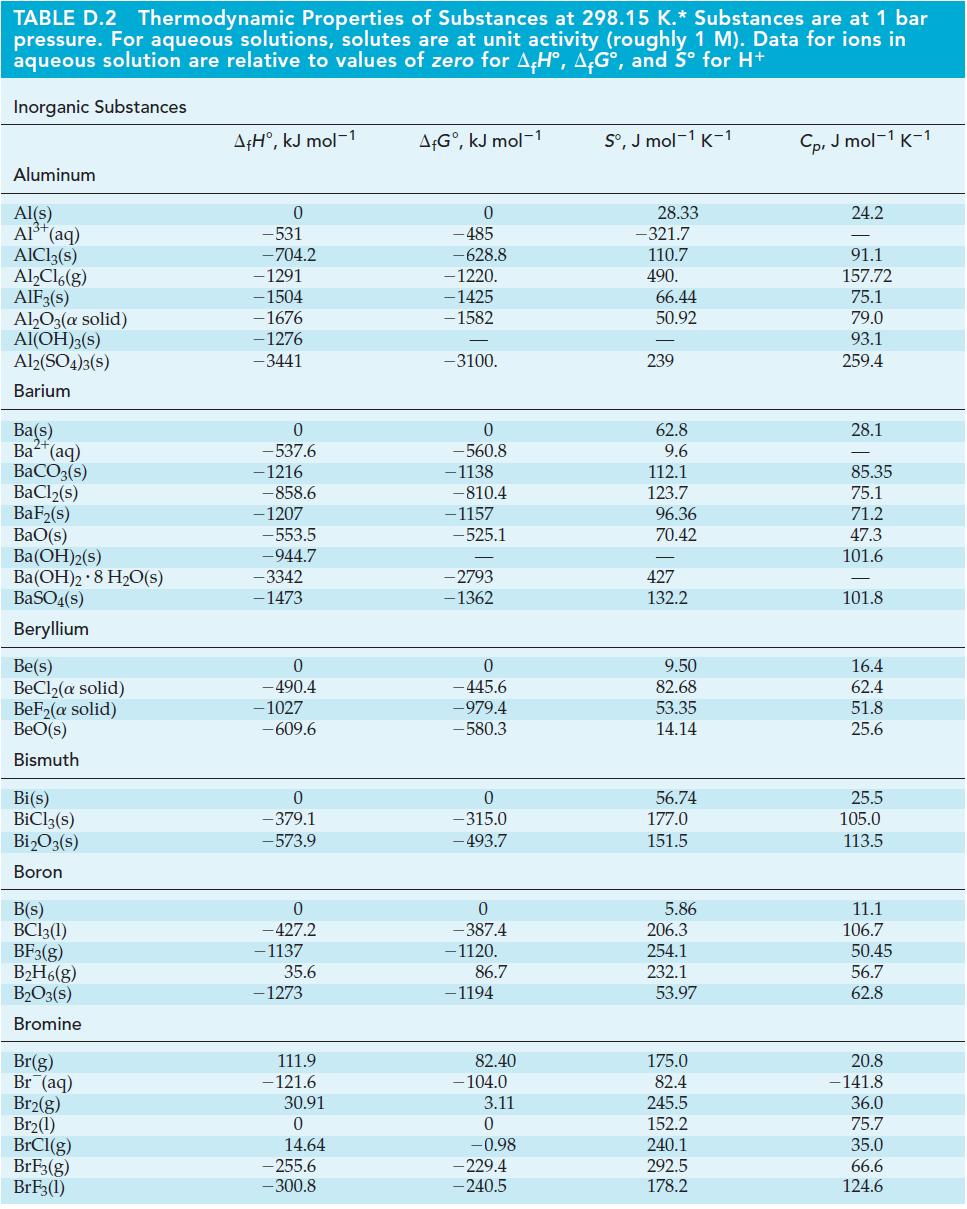
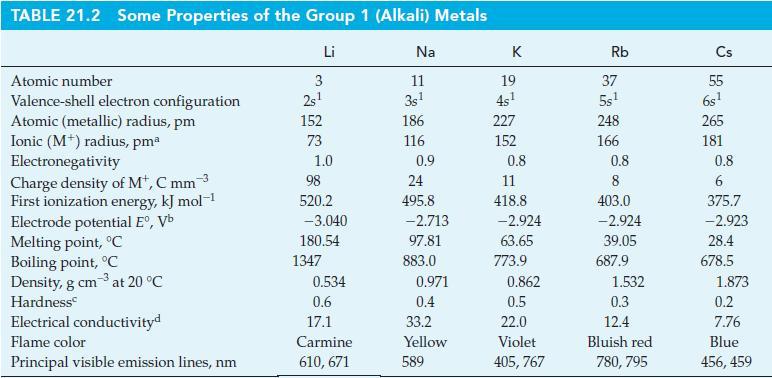
![Acidic solution ([H+] = 1 M): +5 +3 +4 +2 +1 0 - 1 0.803 V 1.065 V 0.996 V 1.591 V 1.766 V -1.87 V 1.42 V](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/5/7/793656722412d5081701257792741.jpg)
![Acidic solution ([H+] = 1 M): +5 +3 +4 +2 +1 0 - 1 1.42 V 1.275 V 0.803 V 1.065 V 0.996 V 1.591 V 1.766 V](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/5/7/975656722f75a4501701257974685.jpg)

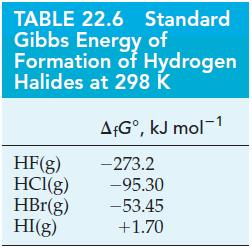
![Acidic solution ([H+] = 1 M): +5 +3 -2 +4 +2 +1 0 - 1 1.275 V 0.803 V 1.065 V 0.996 V 1.591 V 1.766 V-1.87 V](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/4/4/0076566ec67676891701244007198.jpg)