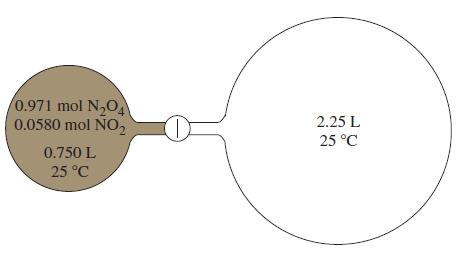
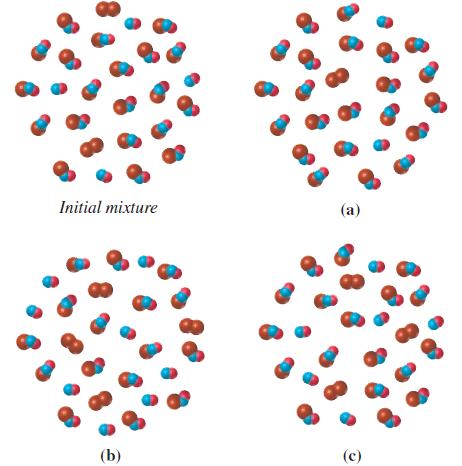
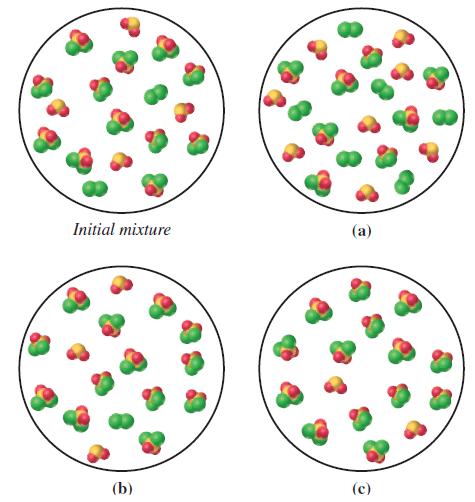
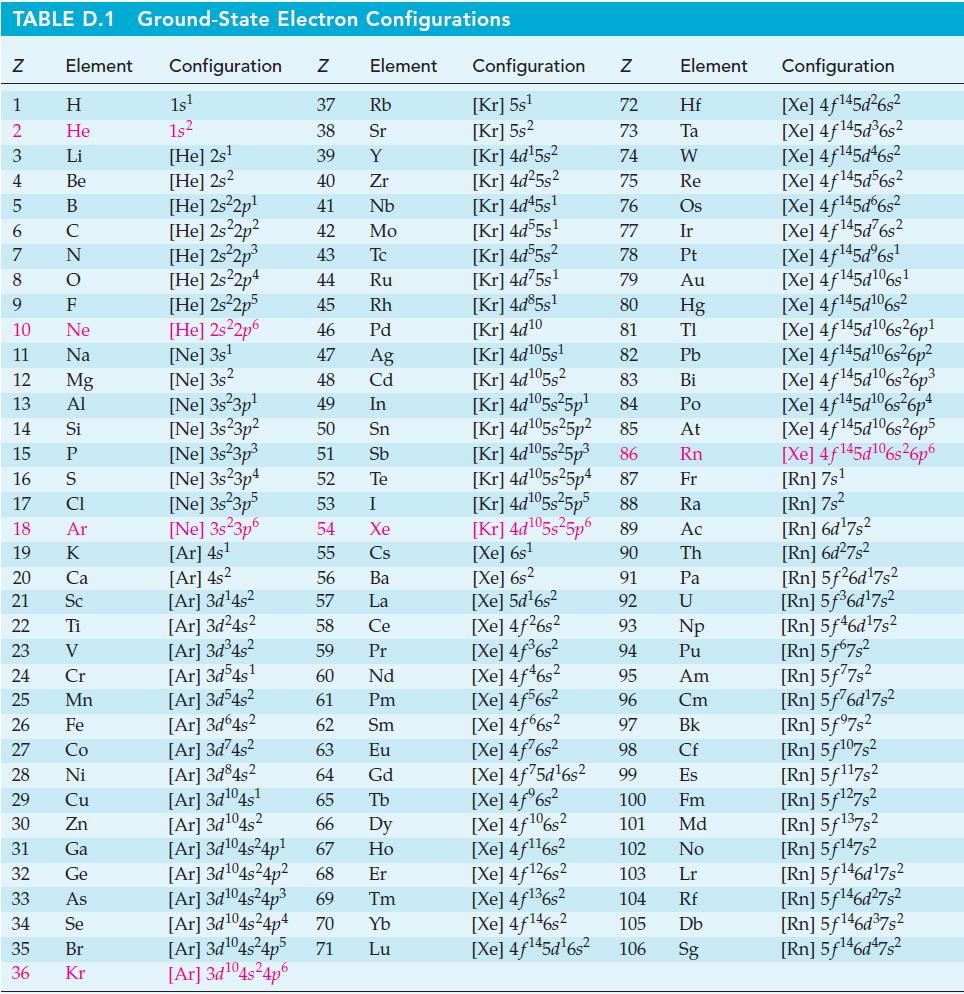
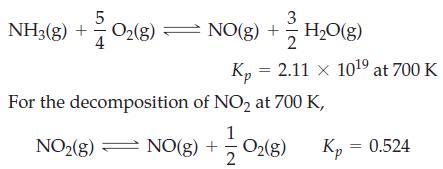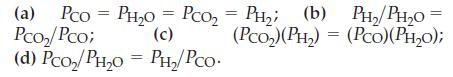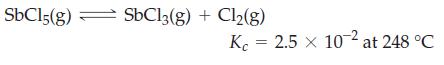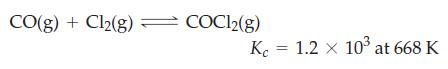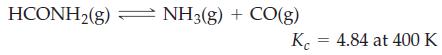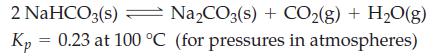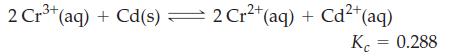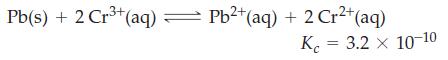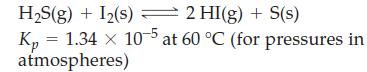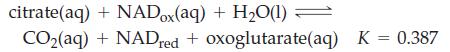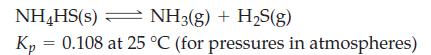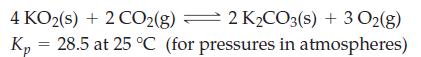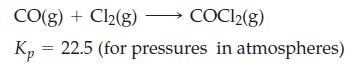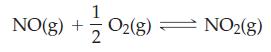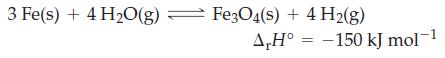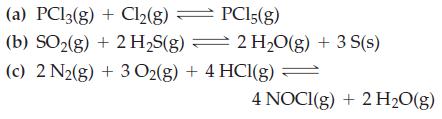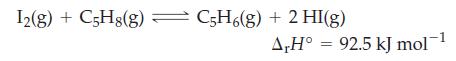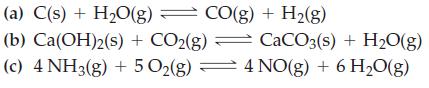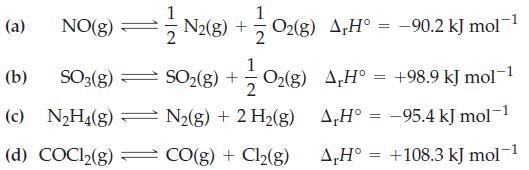![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


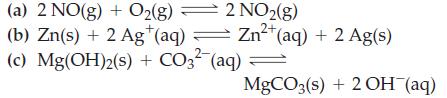
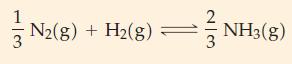
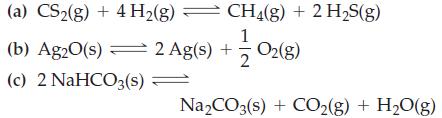
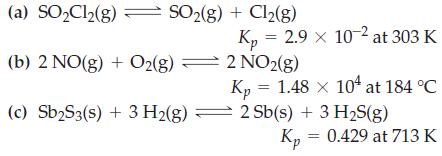
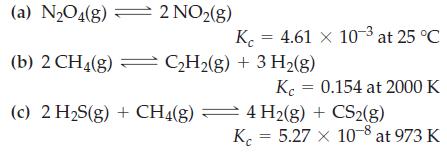
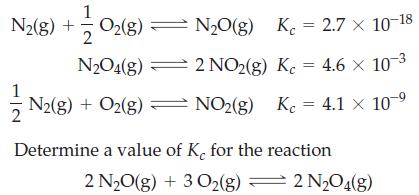
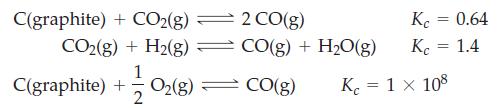
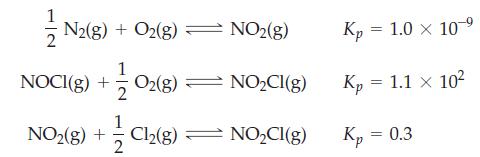
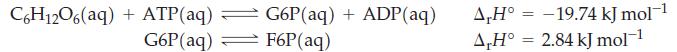
![Fe(OH)3 + 3H*(aq) Fe+ (aq) + 3HO(1) = K = 9.1 x 10 and compute the equilibrium concentration for [Fe+] at](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/4/7/4/598655b2ee6cd4111700474598498.jpg)