![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


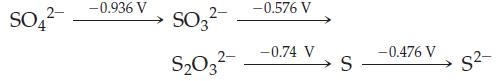
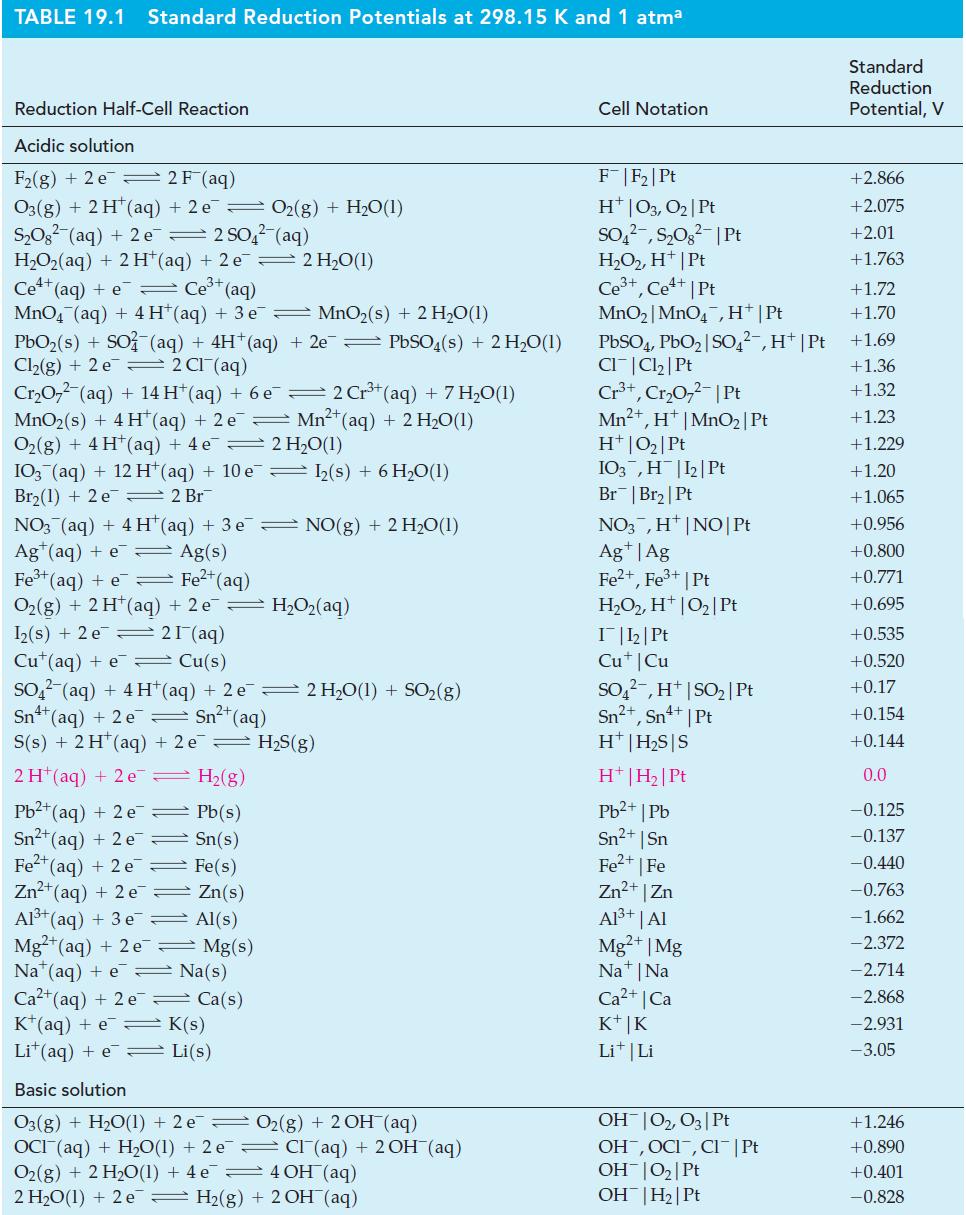
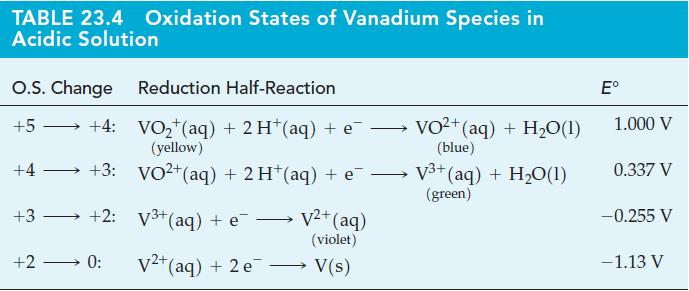
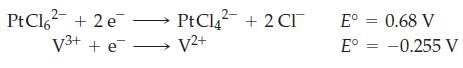
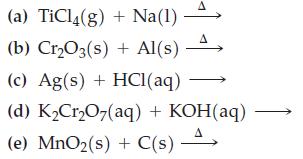
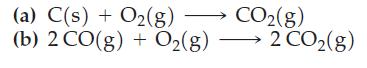
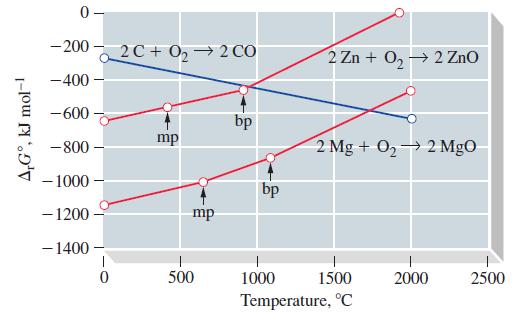
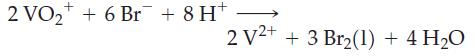
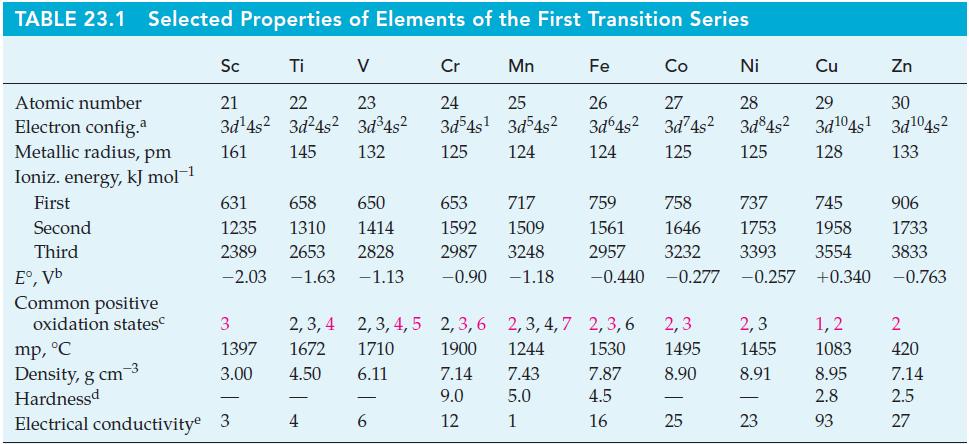
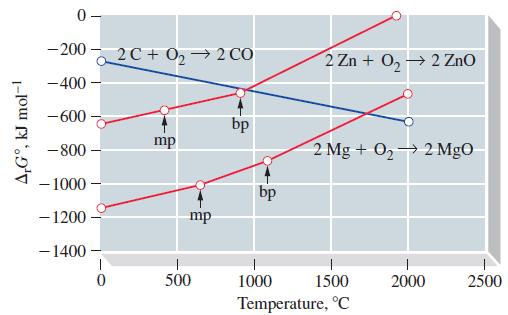
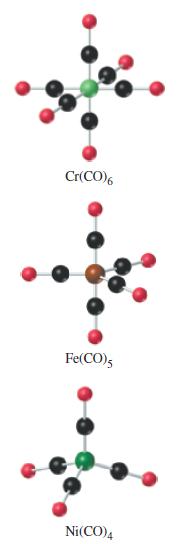
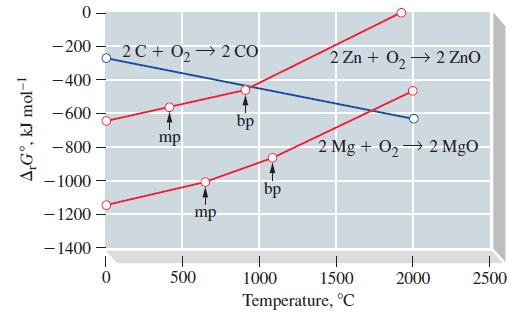
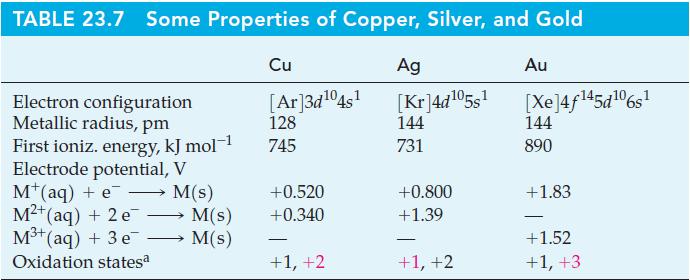
![[Ba+] = [Sr+] = [Ca+] = 0.10 M [CH3COOH] = [CH3COO] = 1.0 M [CrO7] = 0.0010 M Ksp (BaCrO4) = 1.2 10-10 Ksp](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/3/4/5/653656879756d8921701345652214.jpg)