![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![[OCI ], M 0.0040 0.0020 0.0020 0.0020 0.0020 Rate Formation [I], M 0.0020 1.00 0.0040 1.00 0.0020 1.00 0.0020](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/16465641e44d0eff1701060162999.jpg)
![TABLE 20.3 Kinetic Data for the Reaction: 2 HgCl + CO4- 2 Cl + 2 CO + H2Cl Experiment 1 123 2 3 [HgCl], M =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/0/5/3116561c15fe3ed61700905310844.jpg)
![I Time, S 0 25 50 75 100 150 200 250 [A], M 1.00 0.78 0.61 0.47 0.37 0.22 0.14 0.08 || Time, s 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/6466562189edc91d1700927645249.jpg)
![First Experiment [A] = 1.512 M [A] = 1.490 M [A] = 1.469 M Second Experiment [A] = 3.024 M [A] = 2.935 M [A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/8/32965621b49176f61700928325575.jpg)
![Reaction rate Enzyme concentration [E] -](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/0/739656224b3cca871700930739315.jpg)
![[03] [0] rate = k-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/1/252656226b453bc41700931251031.jpg)
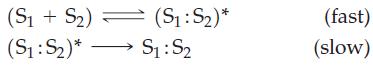
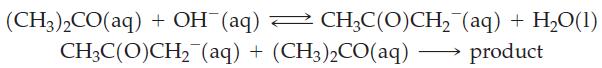
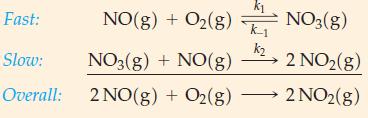
![[A], 1.000 0.800 0.600- 0.400 - 0.200 - 1 2 3 Time, min 4 1 5 1 6](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/1/6956562286f41a791700931684182.jpg)
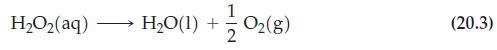
![TABLE 20.1 Decomposition of HO2 Time, s 0 200 400 600 1200 1800 3000 [HO2], M 2.32 2.01 1.72 1.49 0.98 0.62](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/2/12965622a210c72e1700932127357.jpg)
![[H2O2], mol 2.50 - 2.00 - 1.50 - 1.00- 0.50- I 400 800 1200 1600 Time, s 2000 I 2400 2800](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/2/15565622a3b2c1011700932153434.jpg)
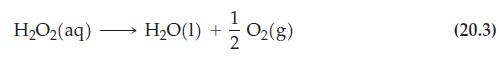
![[OCI ], M [1], M [OH-], MOI, Ms 0.0040 0.0020 0.0020 0.0020 0.0020 0.0020 0.0040 0.0020 0.0020 0.0020 Rate](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/5/8/8446564191c2cc3d1701058842384.jpg)
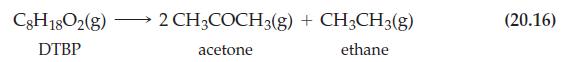
![Expt 1, [B] = 1.00 M Time, min 0 1 5 10 20 [A], M 1.000 10-3 0.951 10-3 0.779 x 10-3 0.607 x 10-3 0.368 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/03265641dc05a8c01701060029838.jpg)
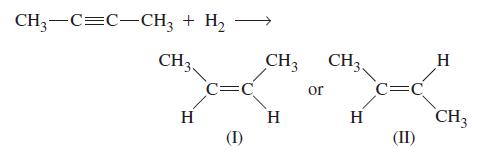
![[A]oX [B]t [B]oX [A]t In- ([B]o [A]o) X kt](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/1/5356564239f633c61701061533524.jpg)

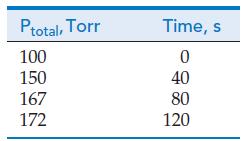
![V = k[E]0[S] KM + [S] (20.36)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/2/393656426f9acee51701062391839.jpg)
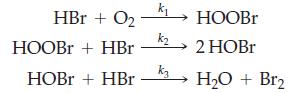
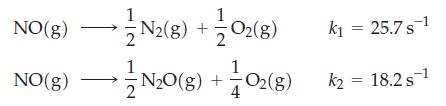
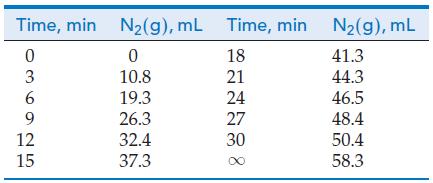
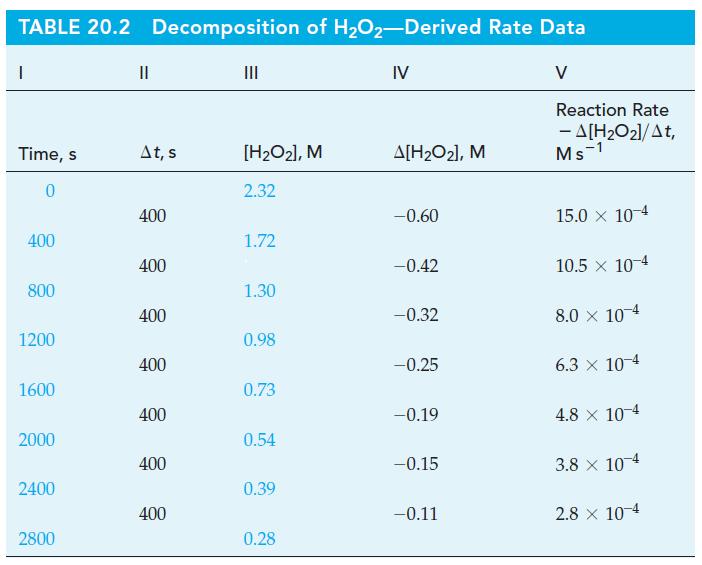
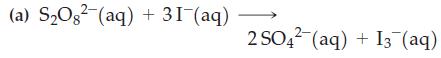
![Experiment 1 1.204 M t = 0 min t = 1.0 min t = 35 min [A] = [A] = 1.180 M [A] = 0.602 M Experiment 2 [A] =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/5/861656434855643b1701065860858.jpg)
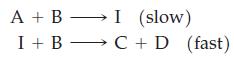
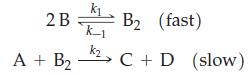
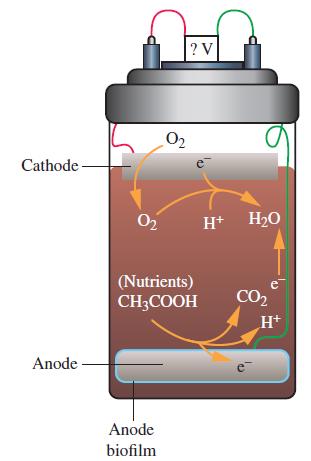
![[CN] = 1.05 M, and Ksp = 8.5 10-7; x for [[Ag(CN)2]]= 0.012 M, [I] = 2.0 M? For Agl, [Ag(CN)], Kf = 5.6 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/4/0/269655f3cad130121.png)